A company called Facedrive has gotten together with the University of Waterloo to create a wearable device to aid in contact tracing. Now that it appears to be operational, it’s ready to sell in collaboration with Microsoft. You remember Microsoft, they helped launched ID2020 back in 2016. Their ex-CEO, Bill Gates, wants to vaccinate the planet.
[Facedrive] is pleased to announce that its contact-tracing platform TraceSCAN has achieved co-sell ready status on the Microsoft Partner Network. Achieving ‘co-sell ready’ status will provide Facedrive TraceSCAN with a significant scaling opportunity by gaining access to Microsoft global customer and partner base. Furthermore, ‘co-sell ready’ status will enable Facedrive and Microsoft teams to collaborate globally on promoting TraceSCAN as a holistic connected health solution powered by Microsoft Azure technology stack. Specifically, Microsoft sales and consulting teams will be able to offer TraceSCAN contact-tracing to their corporate customers as an integrated feature within the enterprise business applications powered by Microsoft products. The greater choice and flexibility provided by being part of the Microsoft Partner’s Network will provide Facedrive TraceSCAN customers with a richer set of options in implementing their contact tracing programs.
It seems that a business deal with Microsoft has been in the works for a while. Considering Gates’ many ties to globalism and this “pandemic”, associations with his former company are worth careful scrutiny.
July 2020, Microsoft announced that TraceSCAN wearables would be available, but distribution would be limited to partners only, for now. This was a sort of soft launch for the product. In September, commercial distribution of the the tracking units started.
December 2020, TraceSCAN received Federal certification from Innovation, Science and Economic Development of Canada (ISED). This used to be known as Industry Canada.
Facedrive appears to incorporate Artificial Intelligence (or AI) into its platform. The company claims that this will assist in forecasting the spread of COVID-19 and predicting any further outbreaks of the virus. In a sense, this device on your wrist would be used to help drive new modelling to make predictions for further lockdowns and martial law.
The AI algorithms will help detect of infected individuals that have not been in direct contact with a positive case but might have been a 2nd or 3rd-degree contact. As with everything, the devil’s in the details, and we would have to know what assumptions and calculations are being made.
The creepiness factor keeps going from there. TraceSCAN’s contact tracing wearables are also a means to track and trace children (even very young children) in their daily movements. Of course, this is being sold as safety and security.
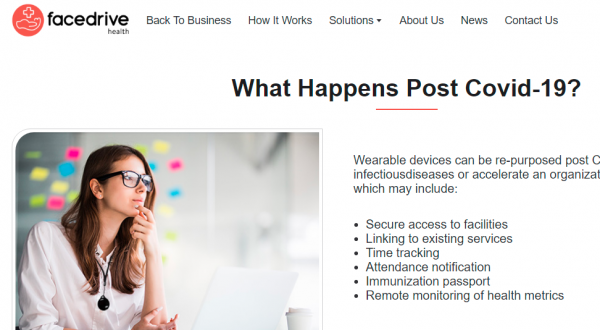
Facedrive itself explains in broad strokes how their technology would work. This amounts to putting a GPS tracker on your wrist, and having your movements and medical conditions tracked. At the same time, this could be done to hundreds, or thousands of other people. This isn’t quite microchipping the cattle, but it’s getting pretty close.
What can this technology be used for? Facedrive gives a list of possibilities:
- Secure access to facilities
- Linking to existing services
- Time tracking
- Attendance notification
- Immunization passport
- Remote monitoring of health metrics
Have to admire how blunt this company is about being able to repurpose their product for more general purposes. At least they don’t lie like the politicians claiming that these trackers will only be limited to this so-called pandemic.
Even back in July 2020, the Ontario Government announced support for this company. As with most things in politics, the magic handshake is needed to get results. From the Provincial database, we are able to see who’s been pulling Ford’s strings this time.
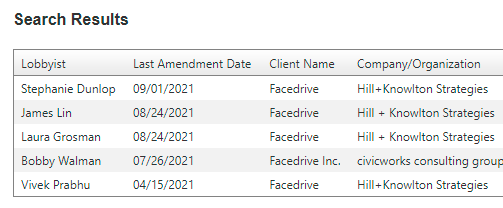
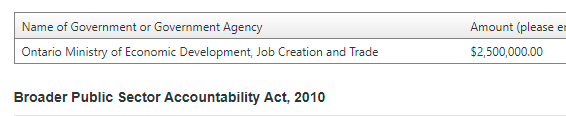
With a quick visit to the Ontario Lobbying Registry, we can see that Facedrive has been active in recent months, using connected lobbyists to get the Government interested in their technology. And it may have helped this company secure a $2.5 million payment from Toronto.
It’s worth a reminder that Microsoft and the Ontario Ministry of Health are both part of the Vaccine Credential Initiative.
Description
VCI is working to enable individuals vaccinated for COVID-19 to access their vaccination records in a secure, verifiable and privacy-preserving way. The Coalition is developing a standard model for organizations administering COVID-19 vaccines to make credentials available in an accessible, interoperable, digital format. empower consumers to conveniently access, store, and share digital COVID-19 vaccination records
Ontario is working towards both a contact tracing system which far expands any legitimate use, and a universal vaccine certification. Anyone remember when this was just 2 weeks to flatten the curve?
Now, who were the people behind the scenes, pulling the strings of Doug Ford? It should surprise no one that the lobbyists involved have ties to the Conservatives both in Ontario, and Federally.
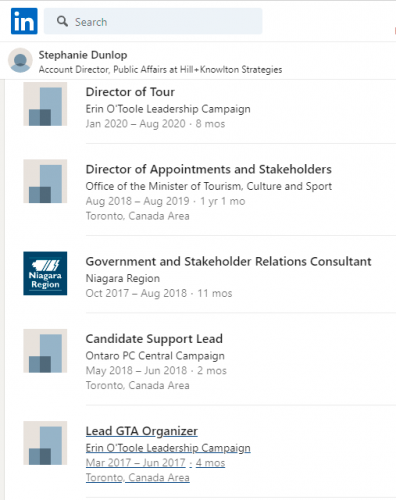
Stephanie Dunlop was involved in both of Erin O’Toole’s runs for the CPC leadership (2017 and 2020). She was also the Candidate Support Lead for the PC Party in 2018. This helped install Doug Ford as Premier of Ontario.
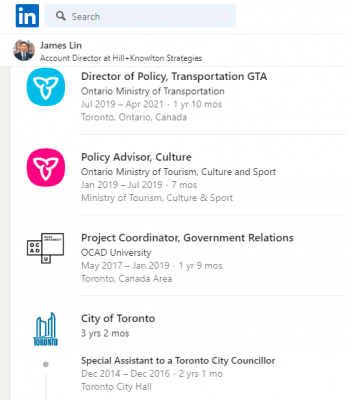
James Lin worked in the Government of Doug Ford, before going over to Hill + Knowlton. He was in the Ministry of Transportation, as a Policy Director. Additionally, he was an Advisor in the Ministry of Tourism, Culture & Sport. He was also involved in the Toronto City Council when Rob and Doug Ford were there. February 2021, she lobbied the Manitoba Government of Brian Pallister over the same contact-tracing platform.
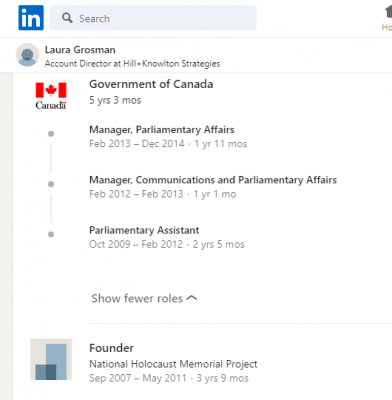
Laura Grossman spent 5 years working for the Government of Canada during the Harper reign.
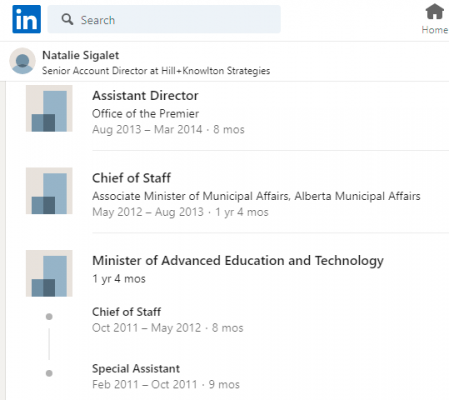
Also worth noting, Natalie Sigalet, a Senior Account Director at the lobbying firm, Hill + Knowlton, has reached out to the Alberta Government of Jason Kenney. She worked in the Office of the Premier of Alberta when Allison Redford was in charge. Presumably, she’s still pretty connected.
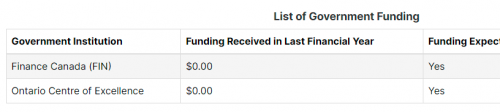
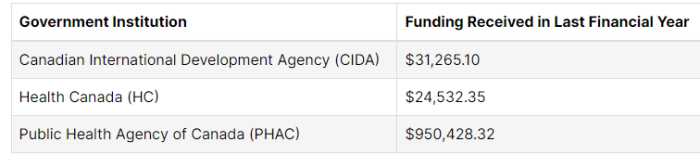
Looking at the Federal Registry, Facedrive is listed there several times. Interestingly, in their 2020 registrations, they list no Government (taxpayer) funding in 2019. However, there is expected to be some coming up from Finance Canada and the Ontario Centre of Excellence. This appears to reference the $2.5 million secured from Ford.
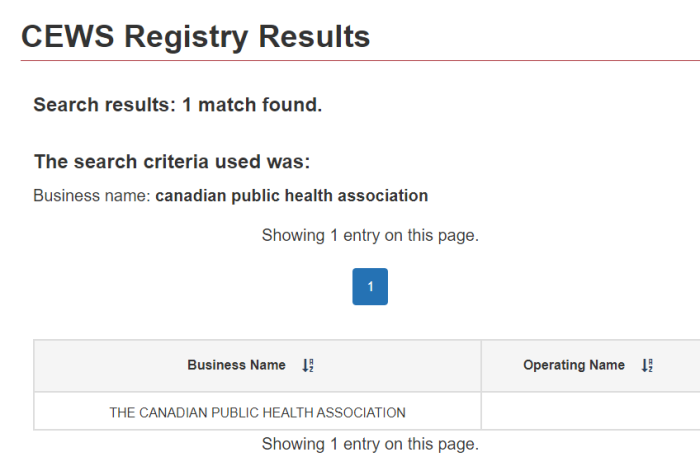
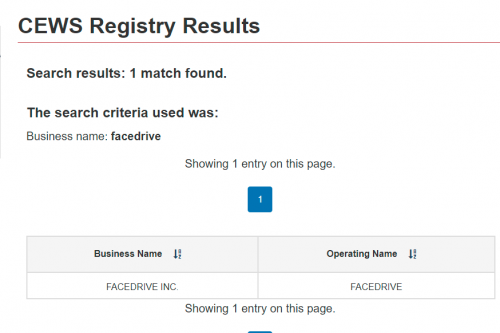
In what should surprise no one, Facedrive has been receiving CEWS, the Canada Emergency Wage Subsidy. Makes sense, as they are very much invested in promoting the pandemic narrative.
In the Azure Marketplace, Microsoft outlines the main goals of this product:
[1] Case Investigation
[2] Contact Tracing
[3] Contact Support
[4] Self Quarantine
Now all of this may sound harmless enough, especially since the self-quarantine is recommended. However, what happens when it becomes mandatory, and wearing this device isn’t a choice? Also, who will be monitoring this system, and what teeth will there be?
This system is just a few short steps away from becoming a Government run chipping and monitoring system. While this may sound hyperbolic, consider where we were even a year ago.
From the looks of things, Microsoft will be used as a hosting platform for which Facedrive is able to launch its product on a much larger scale. However, MS is also eligible to sell units of TraceSCAN under the terms of the arrangement with Facedrive. Of course, that leads to all kinds of privacy and security issues, including who will have access to this data.
And a serious question: what happens if the hosting or management of this system (or part of it) gets sold or outsourced to someone else? What privacy considerations will there be?
Just looking at the products and services offered by Azure, it includes: AI, analytics, blockchain and mixed reality. For people who value any semblance of bodily autonomy and privacy, this needs to be seriously looked into before ever signing on.
And no, this isn’t something new. Even in April 2020, the early days of this psy-op, Microsoft had partnered with the University of Washington. How strange that tracking people was their immediate response.
(1) https://www.youtube.com/watch?v=KX_vdNM33Ug&
(2) https://id2020.org/alliance
(3) https://health.facedrive.com/
(4) https://health.facedrive.com/press-release/facedrives-tracescan-achieves-co-sell-ready-status-with-microsoft/
(5) https://health.facedrive.com/press-release/facedrives-tracescan-wearables-app-now-available-on-microsoft-store-for-partners/
(6) https://health.facedrive.com/press-release/tracescan-starts-shipping-wearable-devices/
(7) https://health.facedrive.com/press-release/facedrive-healths-contact-tracing-technology-tracescan-secures-federal-certification-from-innovation-science-and-economic-development-of-canada-ised/
(8) https://health.facedrive.com/how-it-works/
(9) https://health.facedrive.com/tracescan-ai-platform/
(10) https://health.facedrive.com/school-industry/
(11) https://twitter.com/FacedriveHealth
(12) https://health.facedrive.com/press-release/facedrives-covid-19-tracescan-app-receives-support-of-ontario-government/
(13) https://canucklaw.ca/vaccine-credential-initiative-passports-digital-health-passes-ontario-ford/
(14) http://lobbyist.oico.on.ca/Pages/Public/PublicSearch/Default.aspx
(15) https://www.linkedin.com/in/stephdunlop/
(16) https://registry.lobbyistregistrar.mb.ca/lra/reporting/public/registrar/view.do?method=get®istrationId=414590
(17) https://www.linkedin.com/in/jameslin16/
(18) https://www.linkedin.com/in/laura-grosman-7331a28b/
(19) Facedrive Registration Alberta Sheila Wisniewski
(20) https://www.linkedin.com/in/natalie-sigalet-83b5556a/
(21) https://lobbycanada.gc.ca/app/secure/ocl/lrs/do/vwRg?cno=367466®Id=904875
(22) https://apps.cra-arc.gc.ca/ebci/hacc/cews/srch/pub/bscSrch
(23) https://query.prod.cms.rt.microsoft.com/cms/api/am/binary/RWIzL5
(24) Azure Marketplace Facedrive TraceSCAN
(25) https://azure.microsoft.com/en-us/services/
(26) https://www.geekwire.com/2020/uw-microsoft-release-contact-tracing-app-aiming-battle-covid-19-preserving-privacy/