Interim orders
.
30.1 (1) The Minister may make an interim order that contains any provision that may be contained in a regulation made under this Act if the Minister believes that immediate action is required to deal with a significant risk, direct or indirect, to health, safety or the environment.
.
Marginal note: Cessation of effect
(2) An interim order has effect from the time that it is made but ceases to have effect on the earliest of
(a) 14 days after it is made, unless it is approved by the Governor in Council,
(b) the day on which it is repealed,
(c) the day on which a regulation made under this Act, that has the same effect as the interim order, comes into force, and
(d) one year after the interim order is made or any shorter period that may be specified in the interim order.
Section 30.1 of the Canada Food & Drug Act. Here is the Interim Order signed September 16, 2020 by Patty Hajdu. Now, what does the Interim Order actually say?

Application for authorization
.
3 (1) Subject to section 4, an application for an authorization in respect of a COVID-19 drug must be in a form established by the Minister and contain sufficient information and material to enable the Minister to determine whether to issue the authorization, including
.
(a) the applicant’s name and contact information and, in the case of a foreign applicant, the name and contact information of their representative in Canada;
(b) a description of the drug and a statement of its proper name or its common name if there is no proper name;
(c) a statement of the brand name of the drug or the identifying name or code proposed for the drug;
(d) a list of the ingredients of the drug, stated quantitatively;
(e) the specifications for each of the drug’s ingredients;
(f) a description of the facilities and equipment to be used in the manufacture, preparation and packaging of the drug;
(g) details of the method of manufacture and the controls to be used in the manufacture, preparation and packaging of the drug;
(h) details of the tests to be applied to control the potency, purity, stability and safety of the drug;
(i) the names and qualifications of all the investigators to whom the drug has been sold;
(j) a draft of every label to be used in connection with the drug, including any package insert and any document that is provided on request and that sets out supplementary information on the use of the drug;
(k) a statement of all the representations to be made for the promotion of the drug respecting
(i) the recommended route of administration of the drug,
(ii) the proposed dosage of the drug,
(iii) the drug’s indications, and
(iv) the contra-indications and side effects of the drug;
(l) a description of the dosage form that is proposed for the sale of the drug;
(m) evidence that all test batches of the drug used in any studies conducted in connection with the application were manufactured and controlled in a manner that is representative of market production;
(n) in the case of a drug intended for administration to food-producing animals, the withdrawal period of the drug; and
(o) the known information in relation to the quality, safety and effectiveness of the drug.
4 Content
.
4(2) The application must be in a form established by the Minister and contain the following information and material:
(a) the information and material described in paragraphs 3(1)(a) to (d), (f), (j) to (l) and, if applicable, (n);
(b) an attestation, signed and dated by an individual who has authority to bind the applicant in Canada, certifying that the applicant has access to the information referred to in paragraph 3(1)(o) that was submitted to the relevant foreign regulatory authority in order for the foreign drug to be authorized to be sold;
(c) information that demonstrates that the drug is identical to, and is manufactured, prepared and packaged in the same manner as, the foreign drug;
(d) information that demonstrates that the sale of the foreign drug is authorized by the foreign regulatory authority referred to in paragraph (b); and
(e) any labels that are approved by the foreign regulatory authority referred to in paragraph (b) for use in connection with the foreign drug.
Issuance
.
5 The Minister must issue an authorization in respect of a COVID-19 drug if the following requirements are met:
-the applicant has submitted an application to the Minister that meets the requirements set out in subsection 3(1) or 4(2);
-the applicant has provided the Minister with all information or material, including samples, requested under subsection 13(1) in the time, form and manner specified under subsection 13(2); and
-the Minister has sufficient evidence to support the conclusion that the benefits associated with the drug outweigh the risks, having regard to the uncertainties relating to the benefits and risks and the necessity of addressing the urgent public health need related to COVID-19.
To be clear, getting an authorization under this Interim Order isn’t the same thing as having a drug of vaccine getting approved. This authorization is a sort of temporary emergency measure.
https://covid-vaccine.canada.ca/info/pdf/astrazeneca-covid-19-vaccine-pm-en.pdf
https://covid-vaccine.canada.ca/info/pdf/janssen-covid-19-vaccine-pm-en.pdf
https://covid-vaccine.canada.ca/info/pdf/covid-19-vaccine-moderna-pm-en.pdf
https://covid-vaccine.canada.ca/info/pdf/pfizer-biontech-covid-19-vaccine-pm1-en.pdf
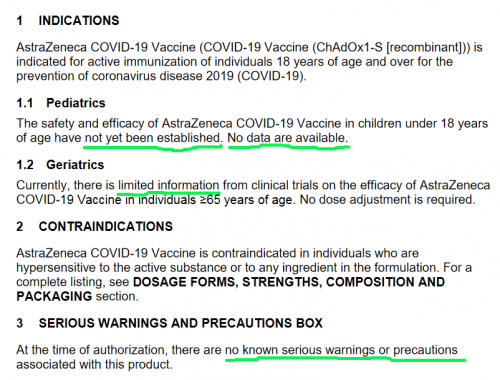





https://covid-vaccine.canada.ca/info/pdf/astrazeneca-covid-19-vaccine-pm-en.pdf

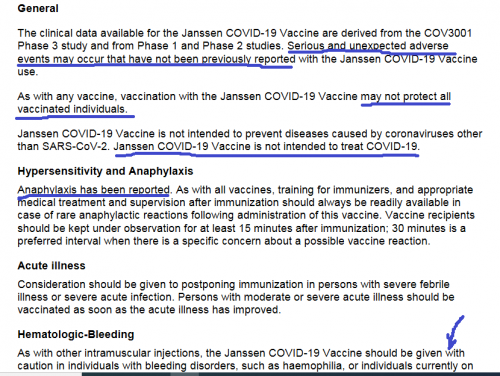





https://covid-vaccine.canada.ca/info/pdf/janssen-covid-19-vaccine-pm-en.pdf







https://covid-vaccine.canada.ca/info/pdf/covid-19-vaccine-moderna-pm-en.pdf




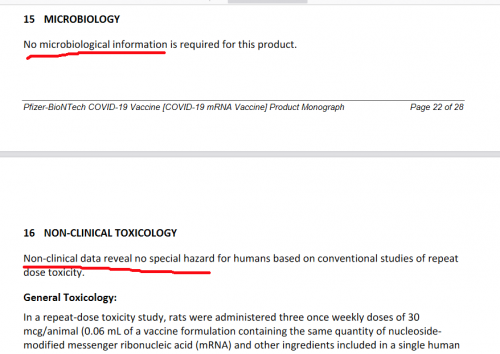


Potassium Chloride is actually used in lethal injections, as it is very effective at slowing down and stopping the heart. But that’s probably just a conspiracy theory.
https://covid-vaccine.canada.ca/info/pdf/pfizer-biontech-covid-19-vaccine-pm1-en.pdf
