Amendment To Original
It seems that many of these powers were already in place from 2002, and then Governor Jeb Bush. However, the vaccine passport ban left these intact. SB 1262, the Emergency Health Powers Act, was passed in the hysteria of terrorism, which the media helped perpetuate. It was (in error), attributed to DeSantis. Instead, he appears to have just left them in place.
There is a separate piece of legislation, SB 6003, to strike “vaccination” out. We’ll have to see how it goes.
Florida Governor Ron DeSantis is frequently hailed as a freedom lover, and a pushback to tyranny in the area. But is that really true? How strong is his resistance?
At no point does DeSantis condemn or criticize these experimental concoctions. He never states that they are not approved, but only allowed because of an FDA Emergency Use Authorization. He never talks about the manufacturers being indemnified from liability.
Granted, he issued a blanket pardon for all of the illegitimate fines and charges handed down for breaching previous draconian Orders, but they should never have been issued in the first place.
For starters, while local officials may be prohibited from imposing mask mandates, there is nothing stopping private businesses from demanding them, even for essential goods.
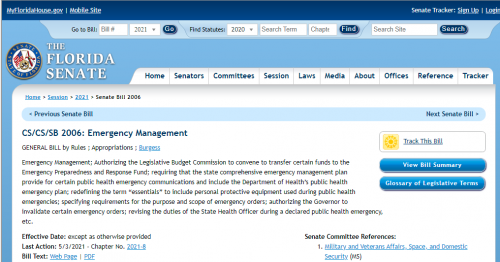
Recently, DeSantis signed SB 2006, which the media claimed would ban “vaccine passports”. While that is true, there were many poison pills left from the Bush era. Either the Governor didn’t fully read the existing Act, or he just didn’t care.
Specifically, still allows the right of the State to impose quarantine measures, similar to what the International Health Regulations call for. It also allows for forced vaccinations. That’s right, a provision was put in to allow for MANDATORY vaccinations “or other treatments”. SB 6003 is in the works to strip vaccination out, but so far, has not been passed.
https://www.flsenate.gov/Session/Bill/2021/2006/BillText/er/HTML is the link, and it seems to be down. So is the general site. Thankfully, it has been archived.
1056 (d) The State Health Officer, upon declaration of a public
1057 health emergency, may take actions that are necessary to protect
1058 the public health. Such actions include, but are not limited to:
1059 1. Directing manufacturers of prescription drugs or over
1060 the-counter drugs who are permitted under chapter 499 and
1061 wholesalers of prescription drugs located in this state who are
1062 permitted under chapter 499 to give priority to the shipping of
1063 specified drugs to pharmacies and health care providers within
1064 geographic areas that have been identified by the State Health
1065 Officer. The State Health Officer must identify the drugs to be
1066 shipped. Manufacturers and wholesalers located in the state must
1067 respond to the State Health Officer’s priority shipping
1068 directive before shipping the specified drugs.
1069 2. Notwithstanding chapters 465 and 499 and rules adopted
1070 thereunder, directing pharmacists employed by the department to
1071 compound bulk prescription drugs and provide these bulk
1072 prescription drugs to physicians and nurses of county health
1073 departments or any qualified person authorized by the State
1074 Health Officer for administration to persons as part of a
1075 prophylactic or treatment regimen.
1076 3. Notwithstanding s. 456.036, temporarily reactivating the
1077 inactive license of the following health care practitioners,
1078 when such practitioners are needed to respond to the public
1079 health emergency: physicians licensed under chapter 458 or
1080 chapter 459; physician assistants licensed under chapter 458 or
1081 chapter 459; licensed practical nurses, registered nurses, and
1082 advanced practice registered nurses licensed under part I of
1083 chapter 464; respiratory therapists licensed under part V of
1084 chapter 468; and emergency medical technicians and paramedics
1085 certified under part III of chapter 401. Only those health care
1086 practitioners specified in this paragraph who possess an
1087 unencumbered inactive license and who request that such license
1088 be reactivated are eligible for reactivation. An inactive
1089 license that is reactivated under this paragraph shall return to
1090 inactive status when the public health emergency ends or before
1091 the end of the public health emergency if the State Health
1092 Officer determines that the health care practitioner is no
1093 longer needed to provide services during the public health
1094 emergency. Such licenses may only be reactivated for a period
1095 not to exceed 90 days without meeting the requirements of s.
1096 456.036 or chapter 401, as applicable.
1097 4. Ordering an individual to be examined, tested,
1098 vaccinated, treated, isolated, or quarantined for communicable
1099 diseases that have significant morbidity or mortality and
1100 present a severe danger to public health. Individuals who are
1101 unable or unwilling to be examined, tested, vaccinated, or
1102 treated for reasons of health, religion, or conscience may be
1103 subjected to isolation or quarantine.
1104 a. Examination, testing, vaccination, or treatment may be
1105 performed by any qualified person authorized by the State Health
1106 Officer.
1107 b. If the individual poses a danger to the public health,
1108 the State Health Officer may subject the individual to isolation
1109 or quarantine. If there is no practical method to isolate or
1110 quarantine the individual, the State Health Officer may use any
1111 means necessary to vaccinate or treat the individual.
1112 c. Any order of the State Health Officer given to
1113 effectuate this paragraph is shall be immediately enforceable by
1114 a law enforcement officer under s. 381.0012.
1115 (e)(2) Individuals who assist the State Health Officer at
1116 his or her request on a volunteer basis during a public health
1117 emergency are entitled to the benefits specified in s.
1118 110.504(2), (3), (4), and (5).
1119 Section 18. Section 381.00316, Florida Statutes, is created
1120 to read:
1121 381.00316 COVID-19 vaccine documentation.—
1122 (1) A business entity, as defined in s. 768.38 to include
1123 any business operating in this state, may not require patrons or
1124 customers to provide any documentation certifying COVID-19
1125 vaccination or post-infection recovery to gain access to, entry
1126 upon, or service from the business operations in this state.
1127 This subsection does not otherwise restrict businesses from
1128 instituting screening protocols consistent with authoritative or
1129 controlling government-issued guidance to protect public health.
1130 (2) A governmental entity as defined in s. 768.38 may not
1131 require persons to provide any documentation certifying COVID-19
1132 vaccination or post-infection recovery to gain access to, entry
1133 upon, or service from the governmental entity’s operations in
1134 this state. This subsection does not otherwise restrict
1135 governmental entities from instituting screening protocols
1136 consistent with authoritative or controlling government-issued
1137 guidance to protect public health.
1138 (3) An educational institution as defined in s. 768.38 may
1139 not require students or residents to provide any documentation
1140 certifying COVID-19 vaccination or post-infection recovery for
1141 attendance or enrollment, or to gain access to, entry upon, or
1142 service from such educational institution in this state. This
1143 subsection does not otherwise restrict educational institutions
1144 from instituting screening protocols consistent with
1145 authoritative or controlling government-issued guidance to
1146 protect public health.
1147 (4) The department may impose a fine not to exceed $5,000
1148 per violation.
1149 (5) This section does not apply to a health care provider
1150 as defined in s. 768.38; a service provider licensed or
1151 certified under s. 393.17, part III of chapter 401, or part IV
1152 of chapter 468; or a provider with an active health care clinic
1153 exemption under s. 400.9935.
1154 (6) The department may adopt rules pursuant to ss. 120.536
1155 and 120.54 to implement this section.
1156 Section 19. Subsection (1) of section 406.11, Florida
1157 Statutes, is amended, and paragraph (c) is added to subsection
1158 (2) of that section, to read:
1159 406.11 Examinations, investigations, and autopsies.—
1160 (1) In any of the following circumstances involving the
1161 death of a human being, the medical examiner of the district in
1162 which the death occurred or the body was found shall determine
1163 the cause of death and certify the death and shall, for that
1164 purpose, make or perform have performed such examinations,
1165 investigations, and autopsies as he or she deems shall deem
1166 necessary or as shall be requested by the state attorney:
1167 (a) When any person dies in this the state:
1168 1. Of criminal violence.
1169 2. By accident.
1170 3. By suicide.
1171 4. Suddenly, when in apparent good health.
1172 5. Unattended by a practicing physician or other recognized
1173 practitioner.
1174 6. In any prison or penal institution.
1175 7. In police custody.
1176 8. In any suspicious or unusual circumstance.
1177 9. By criminal abortion.
1178 10. By poison.
1179 11. By disease constituting a threat to public health.
1180 12. By disease, injury, or toxic agent resulting from
1181 employment.
1182 (b) When a dead body is brought into this the state without
1183 proper medical certification.
1184 (c) When a body is to be cremated, dissected, or buried at
1185 sea.
1186 (2)
1187 (c) A district medical examiner shall assist the State
1188 Health Officer in identifying and reporting deaths upon a
1189 request by the State Health Officer under s. 381.00315.
1190 Section 20. Except as otherwise expressly provided in this
1191 act, this act shall take effect July 1, 2021.
Included in this Bill, SB 2006, are the famous provisions to ban “vaccine passports”, and the text can be found on lines 1122 to 1147. As stated there is a $5,000 (maximum) penalty for breaching this. However, it is not a complete ban, and professions such as health care can still require it.
But that isn’t all. Starting on line 1097
1097 4. Ordering an individual to be examined, tested,
1098 vaccinated, treated, isolated, or quarantined for communicable
1099 diseases that have significant morbidity or mortality and
1100 present a severe danger to public health. Individuals who are
1101 unable or unwilling to be examined, tested, vaccinated, or
1102 treated for reasons of health, religion, or conscience may be
1103 subjected to isolation or quarantine.
A State Health Officer can order a person to be examined, tested, vaccinated, treated, isolated of quarantined for “communicable diseases”. People who refuse, even for valid exemptions, may be quarantined by force. That doesn’t exactly seem consistent with “freedom”. Why is it still there?
1107 b. If the individual poses a danger to the public health,
1108 the State Health Officer may subject the individual to isolation
1109 or quarantine. If there is no practical method to isolate or
1110 quarantine the individual, the State Health Officer may use any
1111 means necessary to vaccinate or treat the individual.
Line 1110 and 1110 state that the State Health Officer may use any means necessary to vaccinate, or otherwise “treat” an individual. What good is it to ban vaccine passports, when the underlying vaccination can still be imposed on a member of the public? What else is in there?
1029 (b) Before declaring a public health emergency, the State
1030 Health Officer shall, to the extent possible, consult with the
1031 Governor and shall notify the Chief of Domestic Security. The
1032 declaration of a public health emergency shall continue until
1033 the State Health Officer finds that the threat or danger has
1034 been dealt with to the extent that the emergency conditions no
1035 longer exist and he or she terminates the declaration. However,
1036 a declaration of a public health emergency may not continue for
1037 longer than 60 days unless the Governor concurs in the renewal
1038 of the declaration.
The State Health Official is an unelected bureaucrat, who has the power to just declare an emergency, and keep it going. Yes, the Governor needs to sign off on renewals past 60 days, but that doesn’t really fix the problem. And who runs Florida anyway, the Governor, or the State Health Officer?
124 specified format; requiring that orders issued by a
125 political subdivision which impose a curfew
126 restricting travel or movement allow persons to travel
127 during the curfew to and from their places of
128 employment; amending s. 377.703, F.S.
Don’t worry about more house arrest (sarcasm). In the event of a forced curfew, people would still be allowed to travel to their jobs. DeSantis won’t PREVENT areas from imposing one, but at least people will still be able to work.
Ron DeSantis is greatly admired in Canada. But is he really the freedom fighter that he claims to be? Why were all of these things left in?
(1) https://www.flsenate.gov/
(2) https://www.flsenate.gov/Session/Bill/2021/2006/BillText/er/HTML
(3) https://archive.is/XCFxp
(4) Wayback Machine Archive
(5) https://www.youtube.com/watch?v=kRFpYmBHzn0
(6) https://www.youtube.com/watch?v=8zeL0lVxXms
(7) https://aapsonline.org/press/jebbushlet.htm
(8) https://www.cidrap.umn.edu/news-perspective/2002/04/state-public-health-emergency-bills-getting-favorable-reception
Like this:
Like Loading...