
The Canadian Agency for Drugs and Technologies in Health (CADTH), is a partner of the Ontario Science Table, or OST. However, CADTH is also a working group for the World Health Organization, Health Evidence Network. Now, OST “claims” to be a neutral and independent body giving scientific and medical advice. Question, is CADTH compromised, or can this do really serve 2 (or more) masters?
The Health Evidence Network describes itself in the following way:
Recognizing that public health, health care and health systems policy-makers need access to timely, independent and reliable health information for decision-making, WHO/Europe started HEN in 2003. It acts as a platform, providing evidence in multiple formats to help decision-making.
The Health Evidence Network also claims to be independent, much the way OST does. Interestingly, they always have the exact same recommendations to make.
Previously Theresa Tam got flack for being on a World Health Organization Committee, while simultaneously claiming to represent Canada as the Public Health Officer. It seems these kinds of conflicts of interest are normal, and not the exception.
CADTH, the Canadian Agency for Drugs and Technologies in Health, claims to be
an independent, not-for-profit organization responsible for providing health care decision-makers with objective evidence to help make informed decisions about the optimal use of health technologies.
Created in 1989 by Canada’s federal, provincial, and territorial governments, CADTH was born from the idea that Canada needs a coordinated approach to assessing health technologies. The result was an organization that harnesses Canadian expertise from every region and produces evidence-informed solutions that benefit patients in jurisdictions across the country.
CADTH claims to be independent, just like OST claims to be independent. The WHO Health Evidence Network also says that it’s an independent entity. Keep that in mind, as it will become important later on. Now, who actually runs CADTH?
- David Agnew: held the position of President and CEO of UNICEF Canada, and was the first head of the organization recruited from outside the international development sector. He is the past Chair of Sunnybrook Health Sciences Centre and of Colleges Ontario. He also serves on numerous other boards and committees, including the Toronto Region Immigrant Employment Council, the Council on Foreign Relations’ Higher Education Working Group on Global Issues, the Sichuan University International Advisory Board, the CivicAction Steering Committee and the Canadian Ditchley Foundation Advisory Board. He is a former member of the federal government’s Science, Technology, and Innovation Council, a former director of ventureLAB and the Empire Club of Canada, and has served on the campaign cabinets of the United Way in Toronto and Peel.
- Marcel Saulnier, Associate Assistant Deputy Minister, Strategic Policy Branch, Health Canada
- Western Provinces, Mitch Moneo, Assistant Deputy Minister, Pharmaceutical Services Division, Ministry of Health, British Columbia
- Mark Wyatt, Assistant Deputy Minister, Saskatchewan Ministry of Health
- Territories, Stephen Samis, Deputy Minister, Health and Social Services, Government of Yukon
- Ontario, Patrick Dicerni, Assistant Deputy Minister, Drugs and Devices Division and Executive Officer, Ontario Public Drug Programs
- Atlantic Provinces, Jeannine Lagassé, Associate Deputy Minister of Health and Wellness, Province of Nova Scotia.
- Karen Stone, Deputy Minister of Health and Community Services (NL)
- Health Systems, Dr. Brendan Carr, President and CEO of the Nova Scotia Health Authority
The Board of Directors of CADTH primarily is made up of high level bureaucrats in Canada, such as Associate Deputy Ministers. Far from being independent, this board is in fact connected to Provincial and Federal Governments.

- drugs
- diagnostic tests
- medical, dental, and surgical devices and procedures
CADTH makes recommendations whether to accept certain medical devices and procedures. They also make recommendations on pharmaceuticals. This is interesting, considering that they don’t seem to do any research themselves. In fact, looking up the term “gene therapy” nets a lot of results.
Strange, because aren’t the Pfizer and Moderna mRNA “vaccines” really just a form of gene replacement therapy? It seems this technology has been around for a while.
Although this may seem harmless enough, there is another aspect to what these Provincial bureaucrats are doing. It’s not only that they want to review and make recommendations, but they want to PROMOTE cheap pharmaceuticals as well.
The pan-Canadian Pharmaceutical Alliance (pCPA) is an alliance of the provincial, territorial and federal governments that collaborates on a range of public drug plan initiatives to increase and manage access to clinically effective and affordable drug treatments.
One of pCPA’s key roles is to conduct joint negotiations for brand name and generic drugs in Canada in order to achieve greater value for publicly funded drug programs and patients through its combined negotiating power. Its objectives are to:
(Alberta) Chad Mitchell, Assistant Deputy Minister
(British Columbia) Mitch Moneo, Assistant Deputy Minister (Vice-Chair, Acting)
(Manitoba) Teresa Mrozek, (A) Assistant Deputy Minister
(New Brunswick) Mark Wies, Assistant Deputy Minister
(Newfoundland & Labrador) John McGrath, (A) Assistant Deputy Minister
(Northwest Territories) Derek Elkin, Assistant Deputy Minister
(Nova Scotia) Natalie Borden, Executive Director
(Nunavut) Donna Mulvey, Territorial Director
(Ontario) Patrick Dicerni, Assistant Deputy Minister; Executive Officer
(Prince Edward Island) Lori Ellis, Director of Health Workforce Planning and Pharmacy
(Quebec) Lucie Opatrny, Assistant Deputy Minister
(Saskatchewan) Mark Wyatt, Assistant Deputy Minister (Chair)
(Yukon) Amy Riske, Assistant Deputy Minister
(Federal) Scott Doidge, Director General
Notice anything? Just like with CADTH, the pan-Canadian Pharmaceutical Alliance is also run by top bureaucrats in the Governments. In fact, Mitch Moneo of B.C., and Mark Wyatt of Saskatchewan sit on both groups. the goal of this group is getting cheap, generic drugs available to all Canadians.
Now, these bureaucrats, and their colleagues, are also involved with the Canadian Agency for Drugs and Technologies in Health, which approves drugs, procedures, and medical devices.
And CADTH is a partner of the Ontario Science Table, which is pushing: mass vaccination, drugs for other health issues, mandatory masks, and lockdowns which will drive up the use of internet and virtual health care.
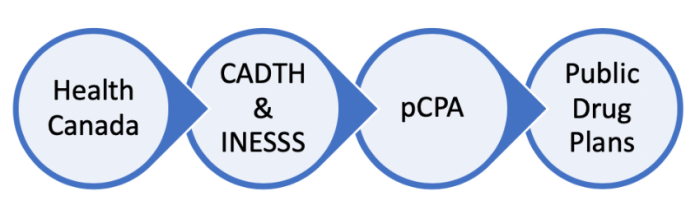
The pCPA site explains the process like this:
Health Canada reviews the drugs, which is not the same as actually testing them. Then CADTH and INESSS (the Quebec counterpart), review it to see if this is a cost effective way to go. Then pCPA tries to negotiate for cheaper and more affordable drug prices. Eventually it gets worked into public and private drug plans.
Back to the original point: the Ontario Science Table claims to be an independent group. But it’s partnered with (among others) CADTH, who plays a major role in advancing big pharma in Canada.
IMPORTANT LINKS
(a) https://www.euro.who.int/en/data-and-evidence/evidence-informed-policy-making/health-evidence-network-hen/technical-members/current-technical-members/canadian-agency-for-drugs-and-technologies-in-health-cadth,-canada
(b) https://covid19-sciencetable.ca/our-partners/
(c) https://www.cadth.ca/about-cadth
(d) https://www.cadth.ca/about-cadth/who-we-are/board-of-directors
(e) https://www.pcpacanada.ca/
(f) https://www.pcpacanada.ca/governance
(g) https://www.pcpacanada.ca/faq
(h) https://www.pcpacanada.ca/about
EARLIER IN THIS SERIES
(a) Michael Warner Financially Benefits From Prolonged Lockdowns
(b) Who Is Ontario Deputy Medical Officer, Barbara Yaffe?
(c) OST, Monopoly From The University Of Toronto Connected
(d) OST, University Of Toronto, Look At Their Members And Partners
(e) OST’s Robert Steiner Claims To Be Behind PHAC Canada Creation
(f) OST’s Kwame McKenzie Headed 2017 UBI Pilot Project
(g) OST UofT Prelude Actually Set Out In May 2019
(h) OST’s Murty Has Tech Firm That Benefits From Lockdowns
(i) Como Foundation Gives Trillium Health Partners $5M
(j) Current PHO Officials Also Sitting On Ontario Science Table
