The public is understandably anxious about whether vaccine manufacturers will be indemnified (legally immune), for the products they sell. It’s a valid question, from a patient perspective, and as a taxpayer.
1. Other Articles On CV “Planned-emic”
The rest of the series is here. Many lies, lobbying, conflicts of interest, and various globalist agendas operating behind the scenes, obscuring the vile agenda called the “Great Reset“. The Gates Foundation finances: the WHO, the US CDC, GAVI, ID2020, John Hopkins University, Imperial College London, the Pirbright Institute, the BBC, and individual pharmaceutical companies. Also: there is little to no science behind what our officials are doing; they promote degenerate behaviour; and the International Health Regulations are legally binding. See here, here, and here. The media is paid off, and our democracy compromised, shown: here, here, here, and here.
2. Email Exchange With Health Canada
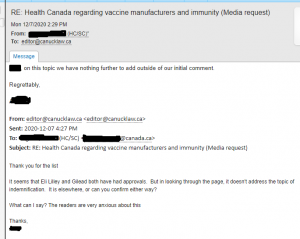

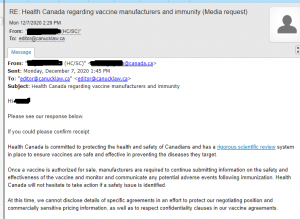
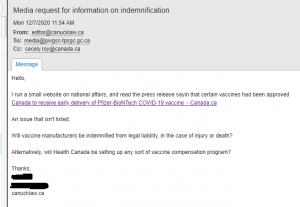
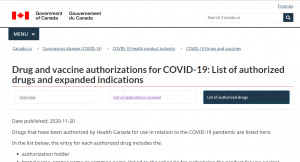
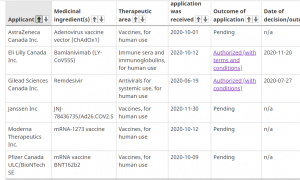
Health Canada was contact specifically about the indemnification of vaccine manufacturers. Above are the responses. However, it’s a bit misleading to say that they can’t release information due to ongoing negotiating. Health Canada wouldn’t even discuss indemnification for Eli Lilly Canada and Gilead Sciences Canada. Both had been settled long ago.
3. Health Canada And Vaccine Regulation



Vaccination is one of the world’s greatest public health achievements. For over 50 years, vaccines have helped prevent and control the spread of deadly diseases and saved the lives of millions of infants, children and adults. For example, there are vaccines for:
-epidemics, such as Ebola
-childhood diseases and debilitating diseases, such as polio
-diseases, such as Yellow Fever, that are common in some travel destinations
-influenza strains that change every year
-preventing or treating cancer
Many vaccines are recommended as part of Canadian public health programs to prevent people from getting diseases. This means that they are given to large numbers of healthy people.
This is why regulating the safety, efficacy and quality of vaccines is of particular importance. There are also reporting systems in place to monitor vaccine safety.
Emergency access to vaccines
In some cases, such as public health emergencies like flu pandemics, special authorizations are used to give emergency access to a vaccine. For example, Health Canada issued an Interim Order in 2009 for the H1N1 pandemic vaccine. The vaccine was developed to protect against the H1N1 pandemic virus. The vaccine contained an inactivated (non-live) version of the H1N1 virus strain recommended by WHO for the manufacture of vaccines during the 2009 flu pandemic.
Worth addressing: in that 2009 Interim Order to approve vaccines for H1N1, Health Canada allowed drugs made by GlaxoSmithKline, (GSK), onto the market that hadn’t been fully tested. GSK was indemnified by the Government. Would it happen here?
Why is it so hard to get a straight answer with this case? Will they be indemnified or not?
