Justin Trudeau, Theresa Tam, Patty Hajdu and others are misrepresenting when they claim that these vaccines have been approved for use. Aside from not really being vaccines, we need to distinguish between 2 things:
(a) Emergency use authorization — deemed to be “worth the risk” under the circumstances, doesn’t have to be fully tested. Allowed under Section 30.1 of the Canada Food & Drug Act.
(b) Approved — Health Canada has fully reviewed all the testing, and steps have been done, with the final determination that it can be used for the general population.
The substances being injected have been authorized for use, because of an Interim Order.
1. Canada Food & Drug Act, Section 30.1
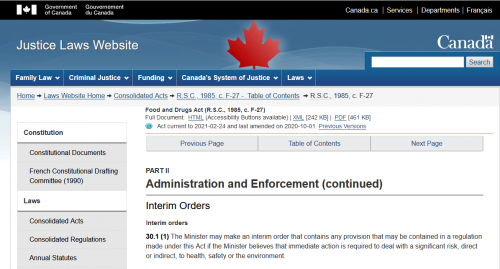
Interim orders
.
30.1 (1) The Minister may make an interim order that contains any provision that may be contained in a regulation made under this Act if the Minister believes that immediate action is required to deal with a significant risk, direct or indirect, to health, safety or the environment.
.
Marginal note: Cessation of effect
(2) An interim order has effect from the time that it is made but ceases to have effect on the earliest of
(a) 14 days after it is made, unless it is approved by the Governor in Council,
(b) the day on which it is repealed,
(c) the day on which a regulation made under this Act, that has the same effect as the interim order, comes into force, and
(d) one year after the interim order is made or any shorter period that may be specified in the interim order.
Section 30.1 of the Canada Food & Drug Act. Here is the Interim Order signed September 16, 2020 by Health Minister Patty Hajdu. This is quite different from having drugs or medical devices being approved through the formal channels. Now, what does that document actually say?
2. September 16 Order From Patty Hajdu

Application for authorization
.
3 (1) Subject to section 4, an application for an authorization in respect of a COVID-19 drug must be in a form established by the Minister and contain sufficient information and material to enable the Minister to determine whether to issue the authorization, including
.
(a) the applicant’s name and contact information and, in the case of a foreign applicant, the name and contact information of their representative in Canada;
(b) a description of the drug and a statement of its proper name or its common name if there is no proper name;
(c) a statement of the brand name of the drug or the identifying name or code proposed for the drug;
(d) a list of the ingredients of the drug, stated quantitatively;
(e) the specifications for each of the drug’s ingredients;
(f) a description of the facilities and equipment to be used in the manufacture, preparation and packaging of the drug;
(g) details of the method of manufacture and the controls to be used in the manufacture, preparation and packaging of the drug;
(h) details of the tests to be applied to control the potency, purity, stability and safety of the drug;
(i) the names and qualifications of all the investigators to whom the drug has been sold;
(j) a draft of every label to be used in connection with the drug, including any package insert and any document that is provided on request and that sets out supplementary information on the use of the drug;
(k) a statement of all the representations to be made for the promotion of the drug respecting
(i) the recommended route of administration of the drug,
(ii) the proposed dosage of the drug,
(iii) the drug’s indications, and
(iv) the contra-indications and side effects of the drug;
(l) a description of the dosage form that is proposed for the sale of the drug;
(m) evidence that all test batches of the drug used in any studies conducted in connection with the application were manufactured and controlled in a manner that is representative of market production;
(n) in the case of a drug intended for administration to food-producing animals, the withdrawal period of the drug; and
(o) the known information in relation to the quality, safety and effectiveness of the drug.
This may be nitpicking, but notice that the Order doesn’t say that the drug has to be safe. It only states that the “unknown information” has to be provided.
It also doesn’t specify that the testing has to be completed, or anywhere close to done. In fact, these authorizations can be issued with next to no testing being done.
Yes, a considerable amount of information needs to be provided. But it doesn’t mean that safety — the biggest issue — has to be conclusively established. The standard is much lower.
4 Content
.
4(2) The application must be in a form established by the Minister and contain the following information and material:
(a) the information and material described in paragraphs 3(1)(a) to (d), (f), (j) to (l) and, if applicable, (n);
(b) an attestation, signed and dated by an individual who has authority to bind the applicant in Canada, certifying that the applicant has access to the information referred to in paragraph 3(1)(o) that was submitted to the relevant foreign regulatory authority in order for the foreign drug to be authorized to be sold;
(c) information that demonstrates that the drug is identical to, and is manufactured, prepared and packaged in the same manner as, the foreign drug;
(d) information that demonstrates that the sale of the foreign drug is authorized by the foreign regulatory authority referred to in paragraph (b); and
(e) any labels that are approved by the foreign regulatory authority referred to in paragraph (b) for use in connection with the foreign drug.
Issuance
.
5 The Minister must issue an authorization in respect of a COVID-19 drug if the following requirements are met:
-the applicant has submitted an application to the Minister that meets the requirements set out in subsection 3(1) or 4(2);
-the applicant has provided the Minister with all information or material, including samples, requested under subsection 13(1) in the time, form and manner specified under subsection 13(2); and
-the Minister has sufficient evidence to support the conclusion that the benefits associated with the drug outweigh the risks, having regard to the uncertainties relating to the benefits and risks and the necessity of addressing the urgent public health need related to COVID-19.
If the above criteria are met, then the authorization MUST be approved, according to Section 5 of the Order.
To be clear, getting an authorization under this Interim Order isn’t the same thing as having a drug of vaccine getting approved. This authorization is a sort of temporary emergency measure. These are not the same thing, and should not be conflated in any way.
Prohibition – significant difference
.
6 (1) It is prohibited to sell a COVID-19 drug to which an authorization relates if any of the matters referred to in subsection 3(1) or subsection 4(2) — other than in paragraph 3(1)(i) or 4(2)(e), as the case may be — are significantly different from the information or material contained in the application, unless the Minister amends the authorization.
Amendment
(2) The Minister must amend the authorization if the following requirements are met:
.
(a) the holder of the authorization has submitted an application to the Minister to amend it;
(b) the holder has provided the Minister with all information or material, including samples, requested under subsection 13(1) in the time, form and manner specified under subsection 13(2); and
(c) the Minister has sufficient evidence to support the conclusion that the benefits associated with the drug outweigh the risks, having regard to the uncertainties relating to the benefits and risks and the necessity of addressing the urgent public health need related to COVID-19.
Notice that the September 16, 2020 Order keeps referring to this as an “authorization” for drugs. It never says the term “approval”. Why is this? It’s because a temporary authorization and an approval are 2 entirely different animals.
True, both lead to “vaccines” getting put into people’s arms. But they are not the same in terms of standards, testing, length of study, and review.
3. Authorized Despite Testing Deficiencies





https://covid-vaccine.canada.ca/info/pdf/astrazeneca-covid-19-vaccine-pm-en.pdf
https://covid-vaccine.canada.ca/info/pdf/janssen-covid-19-vaccine-pm-en.pdf
https://covid-vaccine.canada.ca/info/pdf/covid-19-vaccine-moderna-pm-en.pdf
https://covid-vaccine.canada.ca/info/pdf/pfizer-biontech-covid-19-vaccine-pm1-en.pdf
Want to know the shortcomings in these “thoroughly tested” vaccines? This page contains information directly from the product information. Why aren’t our so-called opposition parties addressing any of this?
Think that suing the manufacturer will be an option if these “vaccines” harm you? Think again. They are exempt from liability. While an injury compensation program was announced back in December, there have been no details or updates since.
4. Same Deception Problem With Fauci
In this recent interview, Anthony Fauci gets called out by Eugenio Derbez for repeatedly distorting the truth. Fauci tries to conflate vaccines being “approved by the FDA”, and an “Emergency Use Authorization”. They are not the same thing. See here for the full conversation.

F-I-N-A-L-L-Y someone other than me is talking about this bogus “approval”!!!!
The EUA is what has the “vaccine” being jabbed in people all over the world. NO COUNTRY has approved the VACCINE period! Not even the USA or the European Union (EU)! FDA has not approved it and if you call the Pfizer hotline, the recorded message states straight up that its vaccine is “NOT FDA APPROVED”! WHY DOES NO ONE PAY ATTENTION???
“The Pfizer-BioNTech COVID-19 vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 16 years of age and older.”
There’s a lot to cover on this site:
https://canucklaw.ca/vaccines-coronavirus-planned-emic/
https://canucklaw.ca/vaccines-coronavirus-planned-emic-lack-of-testing/
https://canucklaw.ca/vaccines-coronavirus-planned-emic-science/
https://canucklaw.ca/vaccines-planned-emic-modelling-liability/
amazing, more pre-eminent blow that out of the water research at CanuckLaw.ca, all in the wording eh. Nothing like sociopath (people with no conscience) homicide (murderers) genocide maniacs (try the genocide description for United Nations) in government, with the badge of honour as Total genocide, war and treason against the citizen. the reality is, they are not vaccines, they are bio-weapons! 5G and microwave technology, is a bio-weapon, but hey, that is a conspiracy theory aka conspiracy fact, like having an apple a day keeps the doctor away is a conspiracy theory, all suppressed and censored by your friendly neighbourhood globalist protocol enforcer mass media mind control propaganda machine…who hates the truth, in fact, the truth is against their “super imposed hate speech laws” unlike their “editorial opinions”
You can always take a look at:
https://www.battlegroup-301.ca/Civil_Defence/Citizen_Protector_M1A.html
Authorized by WHO? A genocide maniac, that’s WHO? Approval happens after the genocide, hurray, clap our hands, total extinction, that is fantastic!!!!
Testing swabs in the nasal cavity puncturing the blood brain barrier and downloading the toxic package aka vaccine, nano tech, bio-weapon, immune system destruction, any word on that? Yes, ok, a Chinese bio-weapon, a Chinese Doctor, Doctor WHO, not Doctor Who time traveller, doing more business with the Chinese Military for…wait for it…more bio-weapons…masquerading as a vaccine, amazing, with a contaminated Chinese mask, and contaminated test swab into your brain, and the 5G military technology bio-weapon Huawei phone system , only a little systemic racism there eh…amazing, how irresponsible.
then of course our all time favourite goblins in big tech, anti social media, mainstream media, infiltrating government everywhere and making illegal laws against the “Magna Carta” and real Canadians about so called hate speech, and goblins censoring white people, it is all conspiracy fact, not theory. Let’s take a look at who owns, controls, votes, edits and censors, shall we? A little bit of system racism against whitey is there? Hmmmm a little bit of. supremacist and privileged position eh, amazing
Total treason and genocide by people in government eh, hmmmm, amazing, and you thought the Queen is not a royal fraud, wow, or how about the White Holocaust, yeah, are you a holocaust denier?
The government spends about $40 billion a year on welfare for immigrants….
but want to snuff out with systemic racism Canadians over 80 at first, then others to follow, since pensions are too much, defenceless people, along with defenceless unborn by abortion, and mind wiping people with total lies enforced through their paid off mass media, and in the slam if you don’t like it eh, isn’t that interesting eh?
Isolation is trauma based mind control eh? trying to control the official narrative eh, like everything else is dis-informations, yikes, can’t have the truth or any other “editorial opinions” especially if it interferes with some globalist sociopath making billions by genocide, sickness, disease and death, can we, systemic racism eh, systemic genocide eh, systemic treason eh, total war, that’s ok right?
Is anyone here interested in joining a class action? an Indictment? Impeachment? discussing the penalty of treason and genocide? Incompetence in government? A new political party, or how about get rid of political parties all together? How about nation state sovereignty, or personal health sovereignty, or self government, what to do with the foreign controlled enemy combatants, enemies foreign and domestic controlling mass media propaganda, that want’s to see you dead, and certainly for you to never know the truth?
And you will know the truth and the truth will make you free…
Wow Mike that was a mouth full. Lots I’m in agreement but you come off a little humm how do you say crazy? No not crazy but a little eccentric?
Did you notice that the website says APPROVED vaccines now??
But if you go to the individual vaccine it still says authorized. I’m sure they are banking on the fact that most people won’t go further than the first page.