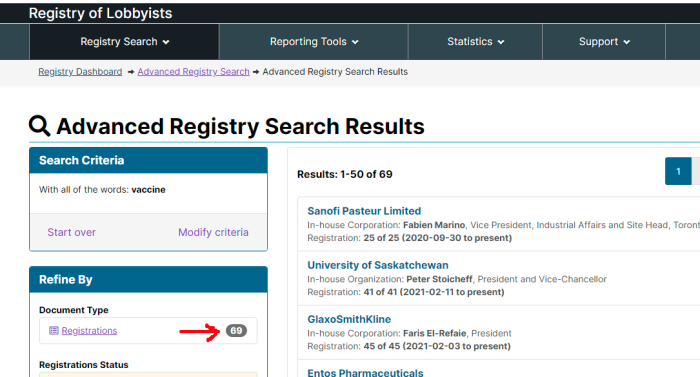
According to the Federal Lobbying Registry, there are 69 ACTIVE registrations that are flagged under the search word of “vaccine”. This includes multiple registrations from the same company, and a few irrelevant hits. Lobbyists aren’t cheap, and there is considerable money tied up in all of this.
Also, what exactly is going on with that proposed vaccine injury compensation program?
1. No Details In Vaccine Injury Program

News release
December 10, 2020 – Ottawa, ON – Public Health Agency of Canada
.
We as Canadians pride ourselves on our commitment to each other. By getting vaccinated, we protect one another and our way of life. Vaccines are safe, effective and one of the best ways to prevent serious illness like COVID-19.
Vaccines are only approved in Canada after thorough and independent review of the scientific evidence. They are also closely monitored once on the market and can quickly be removed from market if safety concerns are identified. Notwithstanding the rigour of clinical trials and excellence in vaccine delivery, a small number of Canadians may experience an adverse event following immunization, caused by vaccines or their administration.
Like any medication, vaccines can cause side effects and reactions. After being vaccinated, it’s common to have mild and harmless side effects — this is the body’s natural response, as it’s working to build immunity against a disease. However, it is also possible for someone to have a serious adverse reaction to a vaccine. The chances of this are extremely rare — less than one in a million — and we have a duty to help if this occurs.
It is for this reason that the Public Health Agency of Canada (PHAC) is implementing a pan-Canadian no-fault vaccine injury support program for all Health Canada approved vaccines, in collaboration with provinces and territories. Building on the model in place in Québec for over 30 years, the program will ensure that all Canadians have to have fair access to support in the rare event that they experience an adverse reaction to a vaccine. This program will also bring Canada in line with its G7 counterparts with similar programs, and ensure the country remains competitive in accessing new vaccines as they become available.
Quick facts
Serious adverse reactions to vaccines are extremely rare. They happen less than one time in a million.
It was announced on December 10, 2020, that a vaccine injury program would be launched in cooperation with the Provinces. That was 2 1/2 months ago, and no details have emerged. Considering that mass vaccination is going on NOW, this is pretty urgent.
In “collaboration with the Provinces” implies that they will have to go along with it as well. If history is any indictation, Federal-Provincial talks go very slowly.
While it’s claimed that vaccines undergo serious testing PRIOR to their approval, that isn’t really the case. Details will be provided in the next section.
2. Vaccines Approved While Still In Testing
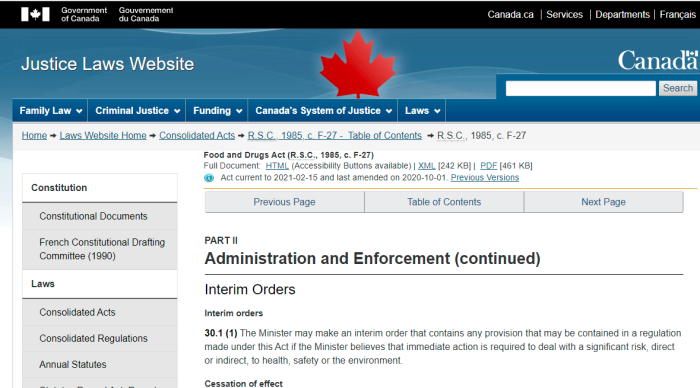
Interim orders
.
30.1 (1) The Minister may make an interim order that contains any provision that may be contained in a regulation made under this Act if the Minister believes that immediate action is required to deal with a significant risk, direct or indirect, to health, safety or the environment.
People naturally assume that a medical product (such as a vaccine), is thoroughly tested prior to being approved. Actually, the Section 30.1 of the Food & Drug Act allows the Health Minister to sign an Interim Order and approve almost anything. And yes, such an Order was signed by Patty Hajdu.
3. Active Lobbying Registrations On “Vaccines”
| COMPANY | LOBBYIST/POSITION/FIRM |
|---|---|
| AstraZeneca Canada Inc. | Jane Chung, President |
| Bayer, Inc. | *Sheamus Murphy, Counsel Public Affairs Inc. |
| Bayer, Inc. | *David Murray, Counsel Public Affairs Inc. |
| Best Medicines Coalition | *William Dempster, 3Sixty Public Affairs Inc. |
| Best Medicines Coalition | Paulette Eddy, Consultant |
| Best Medicines Coalition | Jay Strauss, Consultant |
| Biotecanada | Andrew Casey, President & CEO |
| Canadian Animal Health Institute | Kevin Bosch, Hill+Knowlton Strategies |
| Canadian Medical Association | E. Ann Collins |
| Canadian Medical Association | Timothy Smith, Chief Executive Officer |
| Canadian Pharmacists Association | Annette Robinson, Director |
| Canadian Pharmacists Association | Glen Doucet, Chief Executive Officer |
| Entos Pharmaceuticals | Farid, Faroud, Global Public Affairs Inc. |
| Entos Pharmaceuticals | Conor Mahoney, Global Public Affairs Inc. |
| Entos Pharmaceuticals | *Andrew Retfalvi, Global Public Affairs Inc. |
| Entos Pharmaceuticals | Jay Strauss, Consultant |
| Gavi, the Vaccine Alliance | Ashton Arsenault, Crestview Strategy |
| Gavi, the Vaccine Alliance | Jason Clark, Crestview Strategy |
| GlaxoSmithKline | Faris El-Refaie, President |
| GlaxoSmithKline Inc. | *Bridget Howe, Counsel Public Affairs Inc. |
| GlaxoSmithKline Inc. | *Sheamus Murphy, Counsel Public Affairs Inc. |
| GlaxoSmithKline Inc. | *Ben Parsons, Counsel Public Affairs Inc. |
| GlaxoSmithKline Inc. | *Amber Ruddy, Counsel Public Affairs Inc. |
| Immune Biosolutions | Frédéric Leduc, Président |
| Innovative Medicines Canada | Andrew Balfour, Rubicon Strategy Inc. |
| Innovative Medicines Canada | Pamela Fralick, President |
| Intervac Int’l Vaccine Centre | *Douglas Richardson, McKercher LLP |
| Janssen Inc. (Pharmaceutical Companies of Johnson & Johnson | Jorge Bartolome, President |
| Malaika Vaccine | idee Inyangudor, Wellington Advocacy |
| Medicago Inc. | Ashton Arsenault, Crestview Strategy |
| Medicago Inc. | Jason Clark, Crestview Strategy |
| Medicago Inc. | Danielle Peters, Magnet Strategy Group |
| Medicago Inc. | Patricia Sibal, Crestview Strategy |
| Merck Canada Inc. | Anna Van Acker, President |
| Moderna Therapeutics | Paul Monlezun, Public Affairs Advisors |
| National Ethnic Press and Media Council of Canada | David Valentin, Liaison Strategies |
| Particle Vaccine Canada Ltd. | *Dylan McGuinty, Director |
| *Pfizer Canada ULC | Cole C. Pinnow, President |
| PlantEXT Inc. | *Andre Albinati, Earnscliffe Strategy Group |
| PlantEXT Inc. | *Charles Bird, Earnscliffe Strategy Group |
| PlantEXT Inc. | Craig Robinson, Earnscliffe Strategy Group |
| Sanofi Pasteur Limited | Fabien Marino, Vice President |
| Sanofi Pasteur Limited | *David Angus, Capital Hill Group |
| University of Saskatchewan | *Douglas Richardson, McKercher LLP |
| *University of Saskatchewan | Peter Stoicheff, President and Vice-Chancellor |
| Variation Biotechnologies, Inc. | Francisco Diaz-Mitoma, Consultant |
| Vaxil Biotherapeutics | Lester Scheininger, Barrister and Solicitor |
| Zebra Technologies | *Adria Minsky, Cumberland Strategies |
| Zebra Technologies | Alec Newton, Cumberland Strategies |
- means person has held public office, or organization has former public office holders currently on staff.
4. GSK Lobbyists Worked In Public Offices
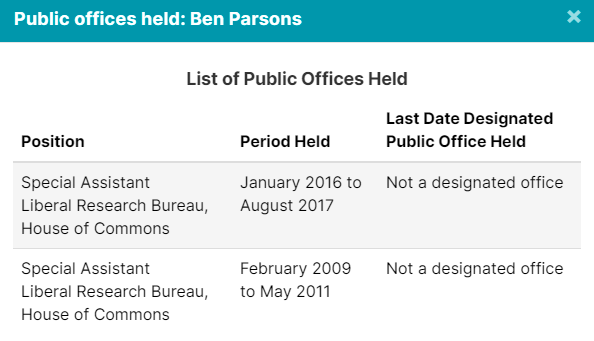
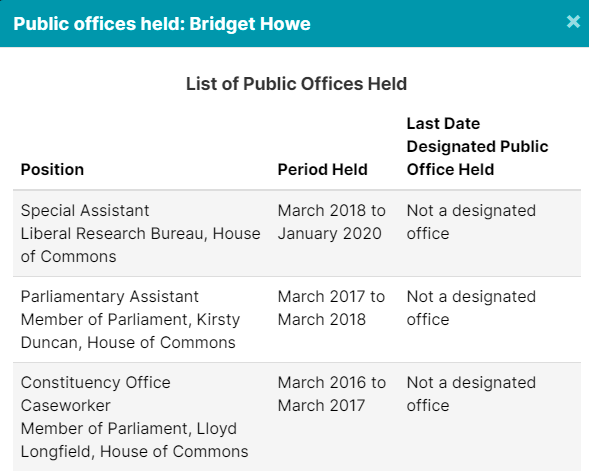
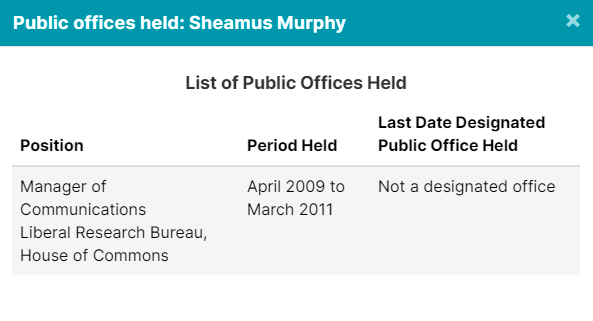
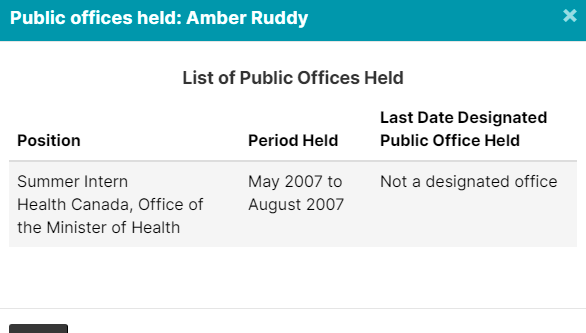
The 4 lobbyists registered to advocate on behalf of GSK, (GlaxoSmithKline), have all held public office in some capacity. But don’t worry, they are probably neutral actors here, and nothing improper will happen.
5. Other Lobbyists Worked In Public Offices
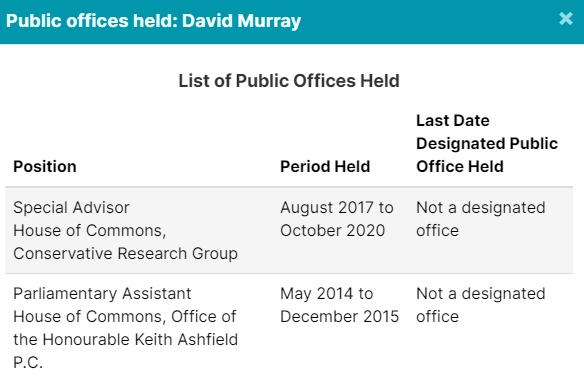
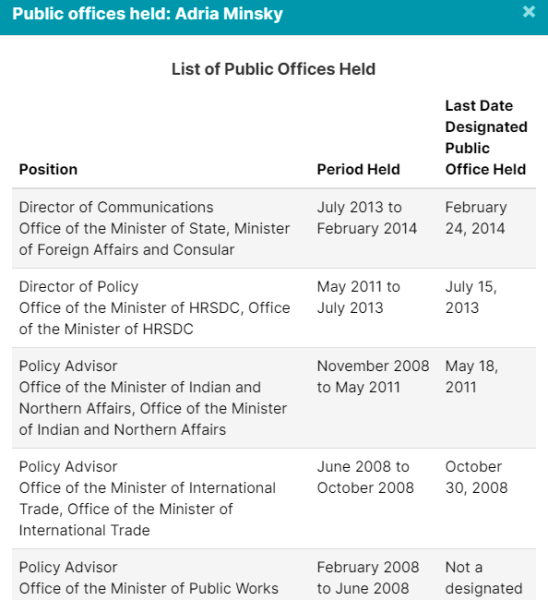
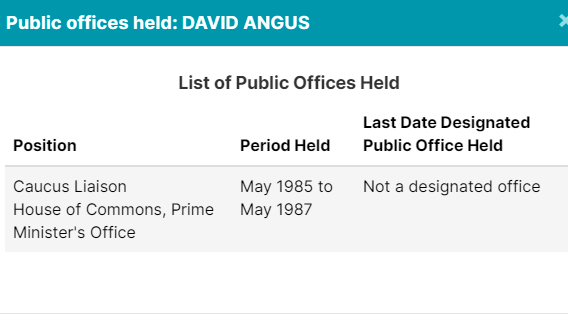
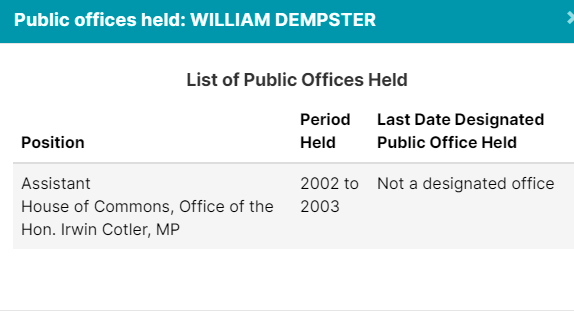
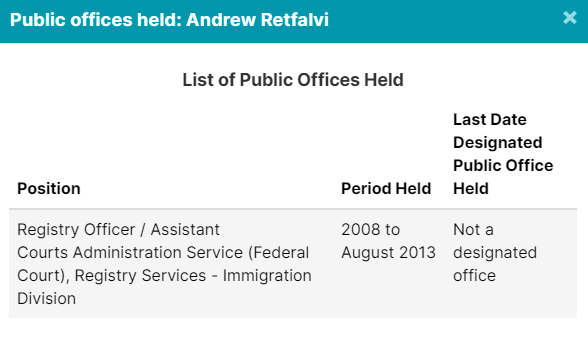
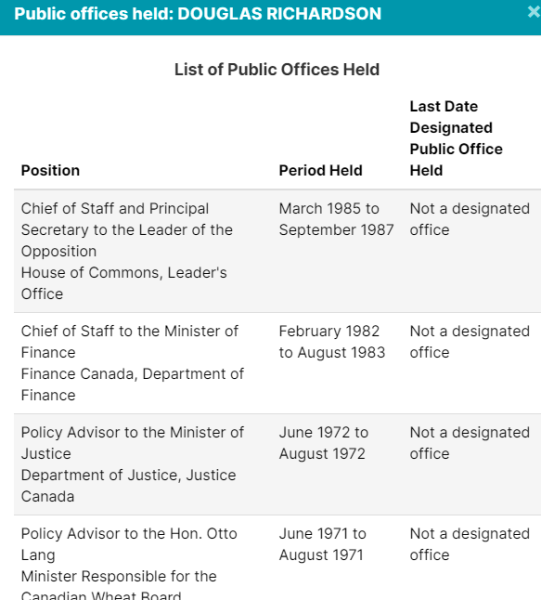
Of course, Crestview Strategy, Ashton Arsenault, Zakery Blais & Jason Clark have all been addressed in previous pieces. Please check them out for more information.
This might also be a good time to bring up the people that have Doug Ford’s attention, Bill 160, Alberta and Quebec lobbying as well.
