At the behest of the World Organization, Governments (including Canada and the Provinces), are playing along with the psy-op that is Covid-19. Now, to anyone who doubts that we are governed by a supra-national body, consider the content below.
1. Other Articles On CV “Planned-emic”
The rest of the series is here. Many lies, lobbying, conflicts of interest, and various globalist agendas operating behind the scenes, obscuring the vile agenda called the GREAT RESET. The Gates Foundation finances: the WHO, the US CDC, GAVI, ID2020, John Hopkins University, Imperial College London, the Pirbright Institute, the BBC, and individual pharmaceutical companies. The International Health Regulations are legally binding. The media is paid off. The virus was never isolated, PCR tests are a fraud, as are forced masks, social bubbles, and 2m distancing.
2. Important Documents
https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19.pdf
WHO Guidelines Classification Of Death
https://www.cpsbc.ca/for-physicians/college-connector/2020-V08-02/04
http://www.bccdc.ca/health-professionals/clinical-resources/covid-19-care/covid-19-testing/viral-testing
http://www.bccdc.ca/Health-Professionals-Site/Documents/COVID19_InterpretingTesting_Results_NAT_PCR.pdf
http://www.cpsa.ca/physicians-notes-basic-principles-on-medical-death-certification/?highlight=covid%20death%20certification
https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-sag-comparison-of-testing-sites-rapid-review.pdf
Alberta Test Comparisons Review
Saskatchewan Death Certificate
Saskatchwan Covid Death Certificate
https://www.saskhealthauthority.ca/news/service-alerts-emergency-events/covid-19/testing-screening-treatment-medical-directives/
Saskatchewan PROV-51-POCT-COVID-Panbio-Antigen-Testing
https://www.gov.mb.ca/health/publichealth/surveillance/covid-19/resources/Notes.html
https://manitoba.ca/asset_library/en/coronavirus/interim_guidance.pdf
Manitoba Interim Guidance
Ontario Public Health Testing FAQ
Ontario Health Covid Lab Testing
http://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/2019_case_definition.pdf
Ontario Covid 2019_case_definition
3. Guidelines Handed Down By WHO

1. PURPOSE OF THE DOCUMENT
This document describes certification and classification (coding) of deaths related to COVID-19. The primary goal is to identify all deaths due to COVID-19.
.
A simplified section specifically addresses the persons that fill in the medical certificate of cause of death. It should be distributed to certifiers separate from the coding instructions.
2. DEFINITION FOR DEATHS DUE TO COVID-19
A death due to COVID-19 is defined for surveillance purposes as a death resulting from a clinically compatible illness, in a probable or confirmed COVID-19 case, unless there is a clear alternative cause of death that cannot be related to COVID disease (e.g. trauma). There should be no period of complete recovery from COVID-19 between illness and death.
.
A death due to COVID-19 may not be attributed to another disease (e.g. cancer) and should be counted independently of preexisting conditions that are suspected of triggering a severe course of COVID-19.
In fairness this may just be extremely poor wording. However, it appears that the default position is to count deaths in confirmed OR PROBABLE cases if the death is from an illness COMPATIBLE WITH Covid-19 symptoms, and we should downplay PREEXISTING CONDITIONS that may have contributed.
As with the diagnosing of cases, there is no requirement to have a positive test. Speaks volumes about how shady this method is.
Now there is the disclaimer that it should not be counted if there is a clear alternative, but this appears to be just an afterthought.
C- CHAIN OF EVENTS
Specification of the causal sequence leading to death in Part 1 of the certificate is important. For example, in cases when COVID-19 causes pneumonia and fatal respiratory distress, both pneumonia and respiratory distress should be included, along with COVID-19, in Part 1. Certifiers should include as much detail as possible based on their knowledge of the case, as from medical records, or about laboratory testing.
D- COMORBIDITIES
There is increasing evidence that people with existing chronic conditions or compromised immune systems due to disability are at higher risk of death due to COVID-19. Chronic conditions may be non-communicable diseases such as coronary artery disease, chronic obstructive pulmonary diseas (COPD), and diabetes or disabilities. If the decedent had existing chronic conditions, such as these, they should be reported in Part 2 of the medical certificate of cause of death.
This guidebook from the World Health Organization is dated April 16, 2020. It defines Covid deaths as “a death resulting from a clinically compatible illness, in a probable or confirmed COVID-19 case”. In other words, it doesn’t have to have CAUSED the death, just be compatible with. Also, a deceased person can be a probable case, meaning no verification is needed that they actually have it.
Now, under WHO’s International Health Regulations, rules handed down are required to be followed by Member States. Let’s see just how well that’s happening.
4. BC College Of Physicians & Surgeons

1. Recording COVID-19 on the medical certificate of cause of death
.
COVID-19 should be recorded on the medical certificate of cause of death for all decedents where the disease caused, or is assumed to have caused, or contributed to death.
2. Terminology
.
The use of official terminology, as recommended by the World Health Organization (i.e. COVID-19) should be used for all certification of this cause of death.
.
As there are many types of coronaviruses it is recommended not to use “coronavirus” in place of COVID-19. This will help to reduce uncertainty for coding and monitoring these deaths which may lead to underreporting.
3. Chain of events
.
Due to the public health importance of COVID-19, when it is thought to have caused or contributed to death it should be recorded in Part I of the medical certificate of cause of death.
Specification of the causal sequence leading to death in Part I of the certificate is also important. For example, in cases when COVID-19 causes pneumonia and fatal respiratory distress, both pneumonia and respiratory distress should be included along with COVID-19 in Part I. Certifiers should include as much detail as possible based on their knowledge of the case, medical records, laboratory testing, etc.
4. Co-morbidities
.
There is increasing evidence that people with existing chronic conditions or compromised immune systems due to disability are at greater risk of death due to COVID-19. Chronic conditions may be non-communicable diseases such as coronary artery disease, COPD, and diabetes or disabilities. If the decedent had existing chronic conditions, such as those listed above, these should be listed in Part II of the medical certificate of cause of death.
Does this look familiar? It is almost word for word identical to that of the World Health Organization, and even explicitly states WHO is used as a reference point.
4. BC Lying About Cases/Testing Errors

Strange question: why would the BC Centre for Disease Control recommend LOWERING the testing threshold for certain people, depending on other factors? Don’t these tests work as advertised? Or is it to confirm a bias when something is suspected?

1. How does the test work?
The NAT works by detecting RNA specific to the SARS-CoV-2 virus that causes COVID-19 infection, after RNA has been extracted from the specimen and then amplified in the laboratory. NATs are typically performed on nasopharyngeal swabs, but the test can also be done on other sample types such as throat swabs, saliva, sputum, tracheal aspirates, and broncho-alveolar lavage (BAL) specimens.
.
The NAT has a high analytical sensitivity (i.e., it works well at detecting the virus when the virus is present). The NAT can potentially detect as few as 10-100 copies of viral RNA per mL in a respiratory sample. Note that this is not the same as clinical sensitivity of NAT for detection of COVID-19 infection, which is unknown at this time (see #5 below).
2. What do the test results mean?
Positive: Viral RNA is detected by NAT and this means that the patient is confirmed to have COVID-19 infection.
A positive NAT does not necessarily mean that a patient is infectious, as viral RNA can be shed in the respiratorytract for weeks but cultivatable (live) virus is typically not detected beyond 8 to 10 days after symptom onset.
Negative: Viral RNA is not detected in the sample. However, a negative test result does not totally rule out COVID-19 infection as there may be reasons beyond test performance that can result in a lack of RNA detectionin patients with COVID-19 infection (false negatives; see below).
Indeterminate: The NAT result is outside the validated range of the test (i.e., RNA concentration is below the limit of detection, or a non-specific reaction), or this might occur when the sample collected is of poor quality (i.e., does not contain a sufficient amount of human cells). Indeterminate results do not rule in or rule out infection.
Overall, clinical judgement remains important in determining the implications of NAT test results, and whether a repeat test is indicated for negative or indeterminate results (for example, if the patient’s recent exposures or clinical presentation suggest COVID-19 infection is likely, diagnostic tests for other respiratory pathogens remain negative, or there is worsening of symptoms). For clinical guidance including testing and specimen collection, please refer to COVID-19 testing guidelines for British Columbia.
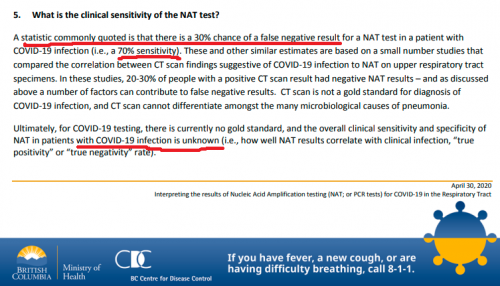
5. What is the clinical sensitivity of the NAT test?
A statistic commonly quoted is that there is a 30% chance of a false negative result for a NAT test in a patient with COVID-19 infection (i.e., a 70% sensitivity). These and other similar estimates are based on a small number studies that compared the correlation between CT scan findings suggestive of COVID-19 infection to NAT on upper respiratory tract specimens. In these studies, 20-30% of people with a positive CT scan result had negative NAT results – and as discussed above a number of factors can contribute to false negative results. CT scan is not a gold standard for diagnosis of COVID-19 infection, and CT scan cannot differentiate amongst the many microbiological causes of pneumonia.
.
Ultimately, for COVID-19 testing, there is currently no gold standard, and the overall clinical sensitivity and specificity of NAT in patients with COVID-19 infection is unknown (i.e., how well NAT results correlate with clinical infection, “true positivity” or “true negativity” rate).
- Can spot 10-100 copies, but that does not equate to infection
- False positives are common
- False negatives are common
- Tests are entirely “open to interpretation”
- 30% false negatives is just a commonly quoted statistic
- CT scan not a gold standard
- CT scan cannot differentiate microbiological causes of pneumonia
Even from the BC CDC’s own document, these tests are worthless, as they cannot be reliably used to detect infection, and are entirely open to interpretation.
5. Alberta Lying About Cases/Testing Errors
After the World Health Organization (WHO) declared COVID-19 a pandemic with increasing mortality, the importance of correctly certifying COVID-19-related deaths is crucial. In view of the public health importance of this infection, when it is thought to have caused death, or is assumed to have caused or contributed to death, it should be recorded in Part I of the medical cause of death. A specification of the causal sequence leading to death (e.g., acute respiratory distress syndrome, or pneumonia) is also important.
The use of official terminology, as recommended by the WHO (i.e., COVID-19), should be used for all certification of this cause of death.
As there are many types of coronaviruses it is recommended not to use “coronavirus” in place of COVID-19. This will help to reduce uncertainty for coding and monitoring these deaths which may lead to underreporting.
If a definite diagnosis cannot be made, but the circumstances are compelling within a reasonable degree of certainty, it is acceptable to report COVID-19 on a death certificate as “presumed” or “probable.”
Alberta also instructs its doctors to list Covid-19 as a cause of death if it’s believed to have caused or contributed to it. There is no requirement to be sure, or even to verify that the person has the virus.
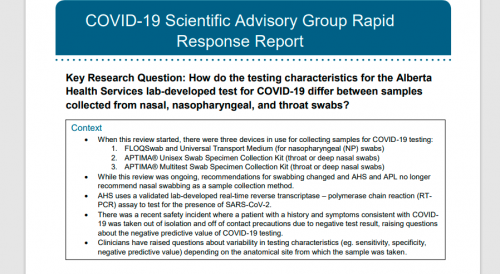
Key Messages from the Evidence Summary
The analytical validity of the lab-developed test used in Alberta is not in question, as confirmatory testing by the Canadian National Microbiology Lab (NML) showed that the Alberta test was 100% accurate, and analytical specificity of PCR testing has been reported to be 100% given the methodology – at least when done during active infection phase.
However, problems with swab collection have been noted, and it is unknown how the anatomical site of sampling and the timing of the sample relative to the disease progression affects the likelihood of RNA detection in a person who is infected with SARS-CoV-2.
There is very limited data regarding the negative predictive values and clinical sensitivity and specificity of commercially developed molecular tests for SARS-CoV-2. What data that exists publicly is a different assay from what is used in Alberta and comparisons should be made with caution.
Studies comparing RNA detection from different sites used samples collected from any of the following sites: nasopharynx, nose, throat, sputum, or bronchoalveolar lavage (BAL) fluid. The evidence was mixed with respect to the superiority (or inferiority) of nasal swabs compared to throat swabs. A small study (n=30) that is ongoing in Alberta indicates that NP and throat swabs may be equivalent while nasal swabs may have lower sensitivity. It is suspected that this is related to a lack of familiarity with deep nasal swab collection and poor collection technique, though this is based on anecdotal evidence.
Recommendations
1. Based on the evidence, false negative samples are infrequent but do occur and would appear to result from insufficient sample collection, emphasizing the importance of proper collection of samples.
2. A program should be devised to identify false negative test results and correlate to clinical cases of COVID-19. To calculate the clinical sensitivity of the test, a consistent case definition and a standard for confirming positive cases will be required. The current lack of a gold standard for confirming positive cases is a significant challenge.
Evidence from grey literature
Instructions for the RT-PCR novel coronavirus diagnostic panel developed by the United States Centers for Disease Control (CDC) highlight the limitations of their assay, specifying that their panel was validated only for respiratory tract specimens and that due to many factors in the sample collection chain, a negative test result should not be used to rule out disease (CDC, 2020a). This document also notes that the predictive values of diagnostic tests are highly dependent on the prevalence and risk of disease (CDC, 2020a). Specifically, false negative test results are more likely when prevalence is high and false positives are more likely when the prevalence is moderate or low (CDC, 2020a), although false positives are rare for RT-PCR based testing when primers and probes are designed appropriately.
This is an extremely important detail that got slipped in. The test is 100% effective — if the person is actually infectious. If they are not, then false positives are quite easy to occur. And this is interesting: they say that false negatives are pretty rare, but B.C. said they could be 30%. Which is it? Moreover, the clinical sensitivity of the test cannot be determined since there is no consistent standard. No “gold standard” anyway.
It speaks volumes about the quality of the test when it’s explained that low prevalence results in more false positives, and vice versa.
So which is it? The quality of the test is not in question, or there are no defined standards to base results on?
6. Sask. Death Certificates, Antigen Tests
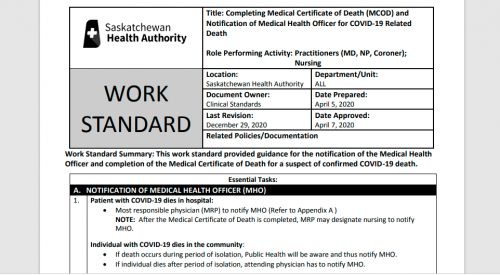
3. RECORDING COVID-19 ON THE MCOD – CAUSE OF DEATH SECTION
COVID-19 should be recorded on the MCOD – Cause of Death section for ALL decedents where the disease caused, or is assumed to have caused, or contributed to death.
4. TERMINOLOGY
The use of official terminology, as recommended by the WHO (i.e. COVID-19) should be used for all certification of this cause of death.
Do not use “coronavirus” in place of COVID-19, as there are many types of coronaviruses. This will help to reduce uncertainty for coding and monitoring these deaths which may lead to underreporting.
5. CHAIN OF EVENTS
When COVID-19 is thought to have caused or contributed to death, it should be recorded in Part 1 of the MCOD – Cause of Death section.
Specification of the causal sequence leading to death in Part 1 of the certificate is also important. For example, in cases when COVID-19 causes pneumonia and fatal respiratory distress, both pneumonia and respiratory distress should be included along with COVID-19 in Part 1. Certifiers should include as much detail as possible based on their knowledge of the case, medical records, laboratory testing, etc.
Here, on a generic model form, is an example of how to certify this chain of events in Part 1:
6. CO-MORBIDITIES
If the decedent had existing chronic conditions, such as those listed below, these should be listed in Part 2 of the MCOD – Cause of Death section.
Chronic conditions may be non-communicable diseases such as coronary artery disease, COPD, and diabetes or disabilities.
There is increasing evidence that people with existing chronic conditions or compromised immune systems due to disability are at greater risk of death due to COVID-19.
Looking at Page 2 of the death certificate, we get this. Looks like it was taken straight from WHO’s guidelines on declaring Covid deaths.

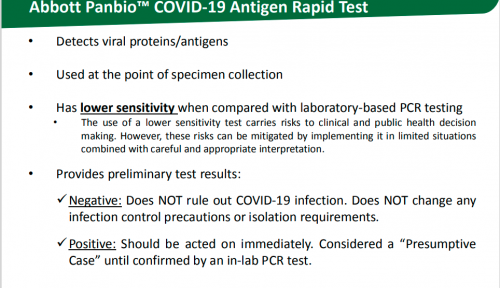
Detects viral proteins/antigens
• Used at the point of specimen collection
• Has lower sensitivity when compared with laboratory-based PCR testing
• The use of a lower sensitivity test carries risks to clinical and public health decision making. However, these risks can be mitigated by implementing it in limited situations combined with careful and appropriate interpretation.
• Provides preliminary test results:
Negative: Does NOT rule out COVID-19 infection. Does NOT change any infection control precautions or isolation requirements.
Positive: Should be acted on immediately. Considered a “Presumptive Case” until confirmed by an in-lab PCR test.
According to the Saskatchewan Health Authority, this antigen test is basically useless. A patient would have to follow up with a PCR test regardless of the outcome.
7. Manitoba Deaths, Cases, PCR “Gold Standard”

Surveillance Case Definition
Cases include both confirmed and probable cases. Surveillance case definitions are provided for the purpose of standardizing case classification and reporting. They are based on evidence, public health response goals, and are subject to change as new information becomes available. Please visit https://manitoba.ca/asset_library/en/coronavirus/interim_guidance.pdf for the most current case definition.
Confirmed case – A person with a laboratory confirmation of infection with the virus that causes COVID-19 performed at a community, hospital or reference laboratory (NML or a provincial public health laboratory) running a validated assay. This consists of detection of at least one specific gene target by a NAAT assay (e.g. real-time PCR or nucleic acid sequencing).
Death due to COVID-19
Source: Adapted from WHO International Guidelines for Certification and Classification (coding) of COVID-19 as a cause of death
.
A death resulting from a clinically compatible illness, unless there is a clear alternative cause of death that cannot be related to COVID disease (e.g. trauma). There should be no period of complete recovery* from COVID-19 between illness and death.
Underlying Illness
Validated algorithms developed by the Canadian Chronic Disease Surveillance System (CCDSS) are used to define the common chronic conditions of COVID-19 cases using administrative health records maintained by Manitoba Health, Seniors and Active Living. (MHSAL)
Under WHO’s rules, even probable cases are considered cases for the purposes of surveillance and reporting. Note: they aren’t even testing for a virus, but for a single gene.

[Page 6]
Laboratory Testing
At present, a validated reverse transcription polymerase chain reaction (RT-PCR) test on a clinically appropriate sample collected by a trained health care provider is the gold standard for the diagnosis of SARS-CoV-2 infection.
[Page 14/15]
Testing Individuals After Death
In the interest of identifying all deaths related to COVID-19 and to better understand the burden of disease in Manitoba, collection of a post-mortem nasopharyngeal (NP) swab for COVID-19 testing should be considered if the following are true:
.
Part A: Prior testing
1) The deceased did not have a NP swab positive for COVID-19 prior to death
OR
2) The deceased did not have two or more NP swabs negative for COVID-19 in the past week
AND
Part B: Symptoms or cause of death
1) Death was preceded by influenza-like illness (ILI), upper or lower respiratory tract infection, or any symptoms compatible with COVID-19, even if very mild
OR
2) Cause of death is unclear
If a previous swab was positive, no further testing is required
It’s a little strange that B.C. claims there is no gold standard for testing, yet Manitoba claims that RT-PCR tests are. Did they not get their stories straight? And this post-death testing comes across as a way to artificially drive up the numbers.
8. Ontario Testing Sensitivity, False Positives
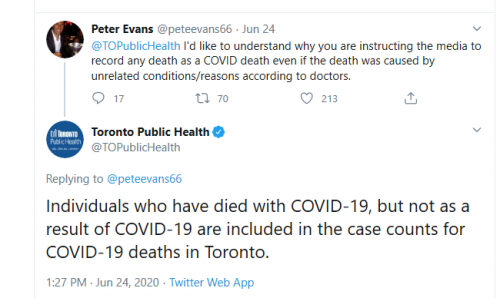

Testing Results and Performance
1. Q. What is the test performance of the PCR assay in use at PHO Laboratory?
A. PHO Laboratory validated the PCR assay currently in use in close collaboration with the National Microbiology Laboratory (NML), Canada’s reference microbiology laboratory. We have excellent concordance with NML from the parallel testing done with them at set up, which included a large number (over 100) and positives (over 20). The sensitivity and specificity of the assay, comparing to NML as the gold standard, is close to 100%.
However, there are many commercial and laboratory developed assays being released and used in Canada, and it is not possible to compare assay performance with every one of them. It is expected there will be some variance in performance if multiple assays are compared to each other, especially around the limit of detection of the individual assays. Parallel testing with the commercial kits in use so far shows similar performance with the assay in use at PHO Laboratory. More information on the testing done at PHO Laboratory from our COVID-19 Test Information Sheet:
https://www.publichealthontario.ca/en/laboratory-services/test-information-index/wuhan-novelcoronavirus
2. Q. What is the positive predictive value of COVID-19 PCR assays?
A. In general, the positive predictive value of COVID-19 PCR assays is excellent, and approaches 100%. At PHO Laboratory, we know this, as we are able to generate viral sequence from samples that are positive provided the viral copy number is not near the limit of detection of the assay.
4. Q. What is the sensitivity, and how often is the COVID-19 test false negative?
COVID-19 Laboratory Testing Q&A 4
A. It is hard to answer this question objectively on how many false negatives there are, as the only way to know is to retest patients who are initially negative, or retest a large number of the same samples with a different assay. Several studies with small sample sizes have been published, and have estimated that the first test done has a sensitivity of 70% to 90% for detecting SARS-CoV-2.
Some interesting questions show up in the Ontario publication. The paper repeatedly claims that the accuracy is close to 100%, but later states that other studies give a sensitivity of 70% to 90%. This would mean a 10% to 30% error. Doesn’t bode well.
9. What Does All This Mean?
The Provinces appear to be following WHO’s directive on deaths, which allows for a “clinically compatible illness” to be the basis for writing Covid-19 on the death certificate. It specifically allows for these certificates to be made based on the flimsiest evidence.
Despite the claims to the contrary, there are some real issues with the PCR and antigen tests. Even taking their words at face value, they are pretty much useless. Rather than them being any sort of “gold standard”, they are open to wide interpretation, and multiple samples may be needed. Keep in mind, they are not testing for an isolated virus, but rather, for a single genetic marker.
Let’s not forget people like Ontario Deputy Medical Officer Barbara Yaffe and Alberta Premier Jason Kenney, who admit potential 50% and 90% errors, respectively. Ontario Health Minister Christine Elliott admitted to fudgind the data when it comes to deaths.
These examples cited are by no means exhaustive. There are surely more documents that contradict the public health narratives on this “pandemic”.
If things really were as bad as advertised, there would be no need to constantly pour out the fear. There’d be no need to “reevaluate” or double check so frequently.
