
1. Other Articles On CV “Planned-emic”
The rest of the series is here. There are many: lies, lobbying, conflicts of interest, and various globalist agendas operating behind the scenes, and much more than most people realize. For examples: The Gates Foundation finances many things, including, the World Health Organization, the Center for Disease Control, GAVI, ID2020, John Hopkins University, Imperial College London, the Pirbright Institute, and individual pharmaceutical companies. It’s also worth mentioning that there is little to no science behind what our officials are doing, though they promote all kinds of degenerate behaviour. Also, the Australian Department of Health admits the PCR tests don’t work, and the US CDC admits there are large problems with current testing.
2. Important Links
https://www.fda.gov/media/134922/download
CDC.serious.testing.problems.July.13
3. CDC: Not Enough Isolates For Testing
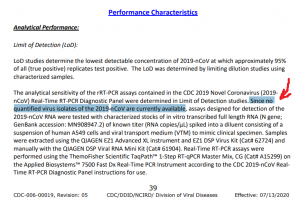
Analytical Performance:
.
Limit of Detection (LoD):
.
LoD studies determine the lowest detectable concentration of 2019-nCoV at which approximately 95% of all (true positive) replicates test positive. The LoD was determined by limiting dilution studies using characterized samples.
.
The analytical sensitivity of the rRT-PCR assays contained in the CDC 2019 Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel were determined in Limit of Detection studies. Since no quantified virus isolates of the 2019-nCoV are currently available, assays designed for detection of the 2019-nCoV RNA were tested with characterized stocks of in vitro transcribed full length RNA (N gene; GenBank accession: MN908947.2) of known titer (RNA copies/µL) spiked into a diluent consisting of a suspension of human A549 cells and viral transport medium (VTM) to mimic clinical specimen. Samples were extracted using the QIAGEN EZ1 Advanced XL instrument and EZ1 DSP Virus Kit (Cat# 62724) and manually with the QIAGEN DSP Viral RNA Mini Kit (Cat# 61904). Real-Time RT-PCR assays were performed using the ThemoFisher Scientific TaqPath™ 1-Step RT-qPCR Master Mix, CG (Cat# A15299) on the Applied Biosystems™ 7500 Fast Dx Real-Time PCR Instrument according to the CDC 2019-nCoV RealTime RT-PCR Diagnostic Panel instructions for use.
Taken at face value, they don’t have enough isolates available, alternative methods would have to be used.
4. CDC Admits False Positives Happen

CDC.serious testing.problems.July.13
Results are for the identification of 2019-nCoV RNA. The 2019-nCoV RNA is generally detectable in upper and lower respiratory specimens during infection. Positive results are indicative of active infection with 2019-nCoV but do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
(Page 3) So a positive result could mean you have the coronavirus, or it could mean something else. That isn’t exactly very helpful.
5. CDC Admits False Negatives Happen

CDC.serious testing.problems.July.13
Results are for the identification of 2019-nCoV RNA. The 2019-nCoV RNA is generally detectable in upper and lower respiratory specimens during infection. Positive results are indicative of active infection with 2019-nCoV but do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results do not preclude 2019-nCoV infection and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
Testing with the CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel is intended for use by trained laboratory personnel who are proficient in performing real-time RT-PCR assays. The CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel is only for use under a Food and Drug Administration’s Emergency Use Authorization.
(Page 3) Okay, so not only will these tests not tell you conclusively that you have the virus, it won’t tell you that you DON’T have it either. False positives and false negatives are bound to happen
5. Extensive List Of Limitations

CDC.serious testing.problems.July.13
• All users, analysts, and any person reporting diagnostic results should be trained to perform this procedure by a competent instructor. They should demonstrate their ability to perform the test and interpret the results prior to performing the assay independently.
• Performance of the CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel has only been established in upper and lower respiratory specimens (such as nasopharyngeal or oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash/aspirate or nasal aspirate).
• Negative results do not preclude 2019-nCoV infection and should not be used as the sole basis for treatment or other patient management decisions. Optimum specimen types and timing for peak viral levels during infections caused by 2019-nCoV have not been determined. Collection of multiple specimens (types and time points) from the same patient may be necessary to detect the virus.
• A false-negative result may occur if a specimen is improperly collected, transported or handled. False-negative results may also occur if amplification inhibitors are present in the specimen or if inadequate numbers of organisms are present in the specimen.
• Positive and negative predictive values are highly dependent on prevalence. False-negative test results are more likely when prevalence of disease is high. False-positive test results are more likely when prevalence is moderate to low.
• Do not use any reagent past the expiration date.
• If the virus mutates in the rRT-PCR target region, 2019-nCoV may not be detected or may be detected less predictably. Inhibitors or other types of interference may produce a false-negative result. An interference study evaluating the effect of common cold medications was not performed.
• Test performance can be affected because the epidemiology and clinical spectrum of infection caused by 2019-nCoV is not fully known. For example, clinicians and laboratories may not know the optimum types of specimens to collect, and, during the course of infection, when these specimens are most likely to contain levels of viral RNA that can be readily detected.
• Detection of viral RNA may not indicate the presence of infectious virus or that 2019-nCoV is the causative agent for clinical symptoms.
• The performance of this test has not been established for monitoring treatment of 2019-nCoV infection.
• The performance of this test has not been established for screening of blood or blood products for the presence of 2019-nCoV.
• This test cannot rule out diseases caused by other bacterial or viral pathogens.
(Page 37/38) A pretty lengthy list: the test itself seems to be plagued by limitations.
6. CDC On Test’s Intended Use
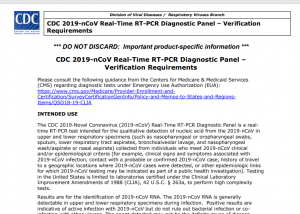
INTENDED USE
The CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel is a realtime RT-PCR test intended for the qualitative detection of nucleic acid from the 2019-nCoV in upper and lower respiratory specimens (such as nasopharyngeal or oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash/aspirate or nasal aspirate) collected from individuals who meet 2019-nCoV clinical and/or epidemiological criteria (for example, clinical signs and symptoms associated with 2019-nCoV infection, contact with a probable or confirmed 2019-nCoV case, history of travel to a geographic locations where 2019-nCoV cases were detected, or other epidemiologic links for which 2019-nCoV testing may be indicated as part of a public health investigation). Testing in the United States is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. § 263a, to perform high complexity tests.
Results are for the identification of 2019-nCoV RNA. The 2019-nCoV RNA is generally detectable in upper and lower respiratory specimens during infection. Positive results are indicative of active infection with 2019-nCoV but do not rule out bacterial infection or coinfection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results do not preclude 2019-nCoV infection and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
Testing with the CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel is intended for use by trained laboratory personnel who are proficient in performing real-time RT-PCR assays. The CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel is only for use under a Food and Drug Administration’s Emergency Use Authorization.
(Page 56) Again, plenty of room for false positives and false negatives from happening. These testing methods can’t even exclude having the virus. They can’t even tell if some other disease is causing the positive result.
7. Testing Has Very Serious Problems
These tests won’t definitively tell people that they have this virus. Nor will they definitively show that a person doesn’t have it. A secondary verification is needed.
The tests also can’t rule out diseases caused by other bacteria or pathogens. So false positives could be cause by other, unrelated illnesses.
The CDC concedes that not enough cell line has been isolated (when at the time of publication), which would further complicate things.
What do Canadian officials have to say about testing and error rates? See the next piece on errors.
-Barbara Yaffe admits up to 50% false positives in virus tests
-Bonnie Henry admits up to 30% false negatives in virus tests
-Bonnie Henry admits high error rates (false positives and false negatives) when it comes to antibody testing.
Do these tests work? Perhaps, but officials admit that the results are highly unreliable. Combine this with the political agendas of many of our leaders, and people have good reason to be skeptical.
