

In 2020, the World Health Organization handed down guidelines that virus isolation was not needed in order to declare a case of Covid-19. In fact, testing positive for a “single discriminatory gene” was sufficient. That raises all kinds of questions.
1. Other Articles On CV “Planned-emic”
The rest of the series is here. Many lies, lobbying, conflicts of interest, and various globalist agendas operating behind the scenes, obscuring the vile agenda called the GREAT RESET. The Gates Foundation finances: the WHO, the US CDC, GAVI, ID2020, John Hopkins University, Imperial College London, the Pirbright Institute, the BBC, and individual pharmaceutical companies. The International Health Regulations are legally binding. The Postmedia empire and the “independent” media are paid off, as are the fact-checkers. The virus was never isolated, PCR tests are a fraud, as are forced masks, social bubbles, and 2m distancing.
2. Important Links
March 2020, WHO Suggest Countries Adopt Own Definitions
Fluoride Free Peel FOI Results
WHO’s March 2020 Testing Guidance
WHO’s September 2020 Testing Guidance
Health Canada Covid-19 Case Definition
BC CDC Covid-19 Case Definition, Testing
Alberta Public Health, Testing Standards
Manitoba Public Health, Cases And Definitions
Ontario Public Health Testing Definitions
BC CDC On Problems With PCR Testing
3. Case Definitions Entirely Subjective

[March 20, Page 1]
Case definitions for surveillance
Case and contact definitions are based on the current available information and are regularly revised as new information accumulates. Countries may need to adapt case definitions depending on their local epidemiological situation and other factors. All countries are encouraged to publish definitions used online and in regular situation reports, and to document periodic updates to definitions which may affect the interpretation of surveillance data.
In their March 20, 2020 guidance, WHO actually suggested countries come up with their own standards and definitions of what a “case” was.
4. WHO Recommends AGAINST Virus Isolation

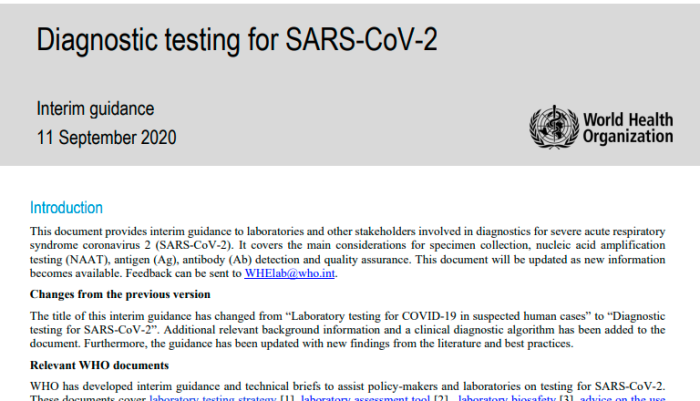
[March 2020, Page 3]
Viral culture
Virus isolation is not recommended as a routine diagnostic procedure.
[September 2020, Page 8]
Viral isolation
Virus isolation is not recommended as a routine diagnostic procedure. All procedures involving viral isolation in cell culture require trained staff and BSL-3 facilities. A thorough risk assessment should be carried out when culturing specimens from potential SARSCoV-2 patients for other respiratory viruses because SARS-CoV-2 has been shown to grow on a variety of cell lines [183].
In both their March 2020, and September 2020 guidance, the World Health Organization explicitly recommends AGAINST virus isolation as a matter of procedure for doing diagnostic testing.
5. Health Canada: Only 1 Gene Needed

[Health Canada, April 2, 2020]
Confirmed
A person with laboratory confirmation of infection with the virus that causes COVID-19 performed at a community, hospital or reference laboratory (NML or a provincial public health laboratory) running a validated assay. This consists of detection of at least one specific gene target by a NAAT assay (e.g. real-time PCR or nucleic acid sequencing).
Health Canada states that detection of even a single gene is sufficient to declare a confirmed case of Covid-19. Nowhere does it say the virus itself must be isolated. Now, the obvious question must be asked: how do we know that this gene is unique? And even that assumes the science is otherwise valid.
6. BC CDC: Only 1 Gene Needed
[BC CDC Guidelines]
Confirmed case
A person with laboratory confirmation of infection with the virus that causes COVID-19 performed at a community, hospital or reference laboratory (NML or a provincial public health laboratory) running a validated assay. This consists of detection of at least one specific gene target by a NAAT assay (e.g. real-time PCR or nucleic acid sequencing).
The BC Centre for Disease Control has the same standards as Health Canada. A single gene target is enough to “confirm” a case.
7. Alberta Health: Only 1 Gene Needed

[Alberta Public Health, Page 3]
Confirmed Case
A person with laboratory confirmation of infection with the virus (SARS-CoV-2) that causes COVID-19 which consists of:
• Detection of at least one specific gene target by nucleic acid amplification tests (NAAT) at a Provincial Public Health Laboratory where NAAT tests have been validated(A)
;
OR
• Confirmed positive result by National Microbiology Lab (NML) by NAAT.
Alberta Health says that even a single gene target is sufficient for a confirmed case.
8. Manitoba Canada: Only 1 Gene Needed

[Manitoba Guidance]
Confirmed case – A person with a laboratory confirmation of infection with the virus that causes COVID-19 performed at a community, hospital or reference laboratory (NML or a provincial public health laboratory) running a validated assay. This consists of detection of at least one specific gene target by a NAAT assay (e.g. real-time PCR or nucleic acid sequencing).
Manitoba Public Health, like the others, writes that even one specific gene target is sufficient. Isolation of the virus is not required at all.
9. Ontario Health: Only 1 Gene Needed

[Ontario Public Health]
Specimens tested using the in-house laboratory developed assay will be tested using the E gene real-time PCR assay, the more sensitive of the two PCR targets.
.
-Specimens with a single target detected (regardless of assay used) will be reported as COVID-19 virus detected, which is sufficient for laboratory confirmation of COVID-19 infection.
-Specimens with no gene target(s) detected in the assay used will be reported as COVID-19 virus not detected.
Ontario Public Health has the same standards as the others: just target a single specific gene. Nothing more is required.
10. BC CDC Admits Tests Don’t Work

1. How does the test work?
The NAT works by detecting RNA specific to the SARS-CoV-2 virus that causes COVID-19 infection, after RNA has been extracted from the specimen and then amplified in the laboratory. NATs are typically performed on nasopharyngeal swabs, but the test can also be done on other sample types such as throat swabs, saliva, sputum, tracheal aspirates, and broncho-alveolar lavage (BAL) specimens.
.
The NAT has a high analytical sensitivity (i.e., it works well at detecting the virus when the virus is present). The NAT canpotentially detect as few as 10-100 copies of viral RNA per mL in a respiratory sample. Note that this is not the same as clinical sensitivity of NAT for detection of COVID-19 infection, which is unknown at this time (see #5 below).
5. What is the clinical sensitivity of the NAT test?
A statistic commonly quoted is that there is a 30% chance of a false negative result for a NAT test in a patient with COVID-19 infection (i.e., a 70% sensitivity). These and other similar estimates are based on a small number studies that compared the correlation between CT scan findings suggestive of COVID-19 infection to NAT on upper respiratory tract specimens. In these studies, 20-30% of people with a positive CT scan result had negative NAT results – and as discussed above a number of factors can contribute to false negative results. CT scan is not a gold standard for diagnosis of COVID-19 infection, and CT scan cannot differentiate amongst the many microbiological causes of pneumonia.
.
Ultimately, for COVID-19 testing, there is currently no gold standard, and the overall clinical sensitivity and specificity of NAT in patients with COVID-19 infection is unknown (i.e., how well NAT results correlate with clinical infection, “true positivity” or “true negativity” rate).
What does this word salad mean? It means that these tests are not able to distinguish between dead genetic material, and live infection. It also means that the BC CDC doesn’t know the error rate. Also, a “statistic commonly quoted” isn’t the same thing as saying THIS virus has such an error rate for testing.
Countries are encouraged to come up with their own case definitions. The virus hasn’t been isolated in advance, and it’s not isolated as a matter of testing. Testing for a single gene target is sufficient, even though the error rate is completely unknown.
People are having their lives and livelihoods destroyed over this, and there is no transparency from public officials, or from the media.
BCPHO Bonnie Henry repeatedly says (regarding group size limits), that none of this is really based on science. And more broadly, this is true. There’s no science behind any of it.

Another great post. Thank you. I also have a document I downloaded about cycling at labs in Ontario. I’ll see if I can post it here in comments after I find it again (can’t upload files here)
https://www.sciencedirect.com/science/article/pii/S138665322030175X
Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories