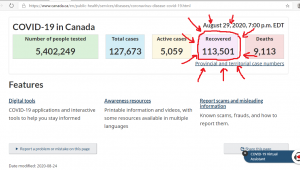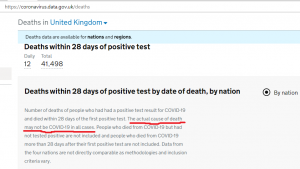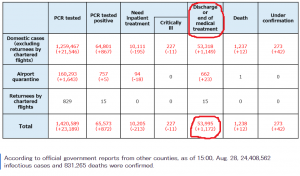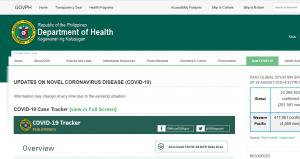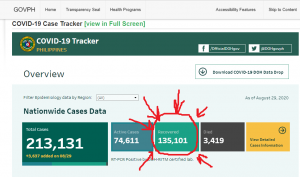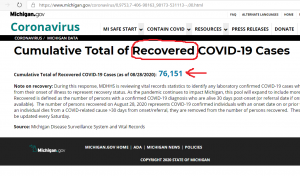
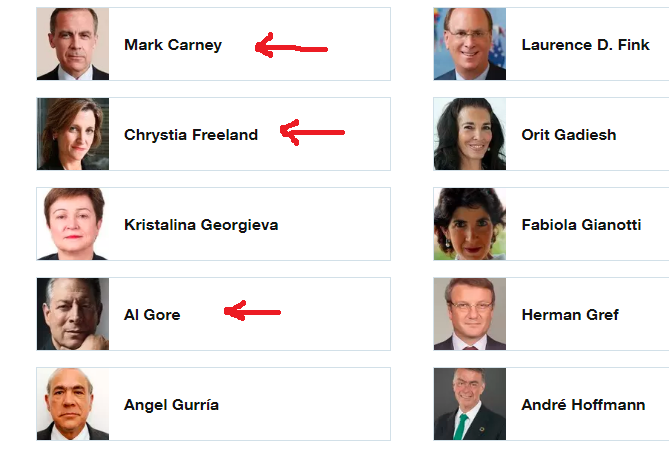
The World Economic Forum, which has: Mark Carney, Chrystia Freeland, and Al Gore as Trustees, it still promoting the “Great Reset” agenda. The person in the top photo self-identifies as Theresa Tam, who is supposed to be the Public Health Officer of Canada.
People seem to think that Canada has control and sovereignty over its own health care and health systems. Let’s put that illusion to rest, once and for all.
1. Other Articles On CV “Planned-emic”
The rest of the series is here. There are many: lies, lobbying, conflicts of interest, and various globalist agendas operating behind the scenes, and much more than most people realize. For examples: The Gates Foundation finances many things, including, the World Health Organization, the Center for Disease Control, GAVI, ID2020, John Hopkins University, Imperial College London, the Pirbright Institute, and individual pharmaceutical companies. It’s also worth mentioning that there is little to no science behind what our officials are doing, though they promote all kinds of degenerate behaviour. Also, the Australian Department of Health admits the PCR tests don’t work, and the US CDC admits testing is heavily flawed.
2. Important Links
(1) https://apps.who.int/gb/bd/pdf_files/BD_49th-en.pdf#page=7
(2) https://www.who.int/news-room/q-a-detail/what-are-the-international-health-regulations-and-emergency-committees
(3) https://archive.is/Ok5jx
(4) https://www.canada.ca/en/health-canada/corporate/about-health-canada/international-activities/international-partners-organizations/world-health-organization.html
(5) https://archive.is/nwz4S
(6) https://apps.who.int/iris/handle/10665/88834
(7) https://archive.is/wwRfk
(8) https://canucklaw.ca/wp-content/uploads/2020/09/ihr.convention.on_.immunities.privileges.pdf
(9) https://apps.who.int/iris/handle/10665/85816
(10) https://archive.is/vJJUE
(11) https://apps.who.int/iris/bitstream/handle/10665/85816/Official_record176_eng.pdf?sequence=1&isAllowed=y
(12) https://www.parl.ca/LegisInfo/BillDetails.aspx?Language=E&billId=1395913&View=5
(13) https://archive.is/YrTHz
(14) https://www.ourcommons.ca/Members/en/votes/38/1/80
(15) https://archive.is/ZbPDU
(16) https://www.who.int/news-room/detail/09-07-2020-independent-evaluation-of-global-covid-19-response-announced
(17) https://archive.is/kofuW
(18) https://www.who.int/about/governance/world-health-assembly/seventy-third-world-health-assembly
(19) https://canucklaw.ca/wp-content/uploads/2020/09/ihr.may_.2020.who_.convention.free_.speech.pdf
(20) https://www.who.int/health-topics/international-health-regulations#tab=tab_1
(21) https://archive.is/OgNwP
(22) https://apps.who.int/iris/bitstream/handle/10665/246107/9789241580496-eng.pdf;jsessionid=8C456867FD2A9E524D1147D63125FD59?sequence=1
(23) https://www.who.int/ihr/about/FAQ2009.pdf?ua=1&ua=1
(24) https://canucklaw.ca/wp-content/uploads/2020/09/ihr.frequently.asked_.questions.pdf
(25) https://apps.who.int/iris/bitstream/handle/10665/69770/WHO_CDS_EPR_IHR_2007.1_eng.pdf?sequence=1
(26) https://www.who.int/ihr/publications/ihrbrief1en.pdf?ua=1
(27) https://canucklaw.ca/wp-content/uploads/2020/09/ihr.brief_.2005.international.obligations.pdf
(28) https://www.who.int/ihr/publications/ihr_brief_no_2_en.pdf?ua=1
(29) https://canucklaw.ca/wp-content/uploads/2020/09/ihr.brief_.2005.reporting.requirements.pdf
(30) https://www.who.int/ihr/publications/ihr_brief_no_3_en.pdf?ua=1
(31) https://canucklaw.ca/wp-content/uploads/2020/09/ihr.brief_.2005.points.of_.entry_.pdf
3. Canada Joins World Health Org. (1949)
Background
-Established in 1946, Canada was the Third Member State to ratify the Constitution on August 29, 1946
-A Canadian Deputy Minister of Health, Dr. Brock Chisholm, became WHO’s first Director General
-Canada’s points of intervention occur during the World Health Assembly, at the Executive Board, Regional Committees and by participating in the work of technical groups; Tropical Diseases Research, Human Reproduction and Child Health and Development. Technical input is with Health Canada
-International Affairs Directorate is the primary contact for WHO in Canada
-The Directorate performs a representation and co-ordination function for the Canadian Health Sector – Health -Canada, other federal agencies, the provinces, universities and the NGO sector
-Support increasing involvement by line branches in the technical work of WHO and its programmes (International Agency on Cancer, International Program on Chemical Safety, etc)
Canada joined the WHO on August 29, 1946.
4. International Sanitary Regulations (1951)

WHO originally adopted the International Health Regulations (IHR or Regulations) as the International Sanitary Regulations in 1951. Article 21 of the WHO Constitution (1948) empowers the World Health Assembly (the main policy-making organ of WHO) to adopt “regulations” concerning, among other things, infectious disease control; and the World Health Assembly adopted the International Sanitary Regulations under this authority in order to consolidate in one instrument the many international sanitary conventions negotiated since the late nineteenth century. [4] WHO changed the name of the Regulations to the IHR in 1969 and last revised them in 1983 when it removed smallpox from the IHR’s list of diseases. Under Article 22 of the WHO Constitution, Assembly-adopted regulations are binding on all WHO member states except those that notify the Director-General of rejection or reservations within a specified time.
The International Health Regulations originally was called the International Sanitary Regulations, and was updated over time. An interesting article on it, by David Fidler.
5. Convention On Immunities & Privileges (1959)

WHA12.41 Convention on the Privileges and immunities of the Specialized Agencies: Specification of Categories of Officials under Section 18 of Article VI of the Convention
The Twelfth World Health Assembly,
.
Considering Section 18 of Article VI of the Convention on the Privileges and Immunities of the Specialized Agencies which requires that each specialized agency will specify the categories of officials to which the provisions of that Article and Article VIII shall apply; and Considering the practice hitherto followed by the World Health Organization under which, in implementing the terms of Section 18 of the Convention, due account has been taken of the provisions of resolution 76 (I) of the General Assembly of the United Nations,
.
1. CONFIRMS this practice; and
2. APPROVES the granting of the privileges and immunities referred to in Articles VI and VIII of the
Convention on the Privileges and Immunities of the Specialized Agencies to all officials of the World Health Organization, with the exception of those who are recruited locally and are assigned to hourly rates.
Eleventh plenary meeting, 28 May 1959 (section 3 of the fourth report of the Committee)
https://apps.who.int/iris/handle/10665/88834
ihr.convention.on.immunities.privileges
Even back in 1959, the World Health Organization saw that its members should enjoy full legal immunity for itself, and its agents. Of course, member states seemed happy to go along with it. Looking through the records though, it seems unclear if Canada has specifically signed on.
6. World Health Assembly (1969, Boston)

WHA22.46 International Health Regulations
The Twenty- second World Health Assembly,
Having considered the recommendations of the Committee on International Quarantine in its fifteenth
report, Volume A, concerning the special review of the International Sanitary Regulations;
Noting that the Committee on International Quarantine reaffirmed the principles laid down in its fourteenth report, Volume II;
1 See Annex 5.
RESOLUTIONS AND DECISIONS 23
Noting also that the comments of Member States were considered by the Committee on International Quarantine at its fifteenth meeting when preparing the draft International Health Regulations to replace the existing International Sanitary Regulations,
1. cor1 ENDS the members of the Committee for their work; and
2. ADOPTS this twenty -fifth day of July 1969 the International Health Regulations annexed to this resolution together with Appendices 1 to 6 concerning the forms and certificates, and the rules applying thereto.’
Handb. Res., 10th ed., 1.3.9.3 Fourteenth plenary meeting, 25 July 1969 (Committee on Programme and Budget, sixth report)
1969 World Health Assembly, Boston.
official records, of WHA (Boston, 1969)
What all of this means is that the Committee on International Quarantine, (a subgroup of WHO), has laid out new guidelines for how to conduct a mass quarantine of people. Canada, as a member of the World Health Organization, is bound by these regulations.
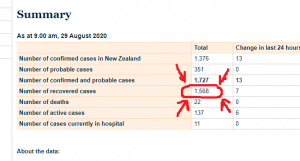
7. New Zealand, Quarantine Act (1983)

If you think this issue is limited to Canada, you would be mistaken. New Zealand also adopted its version of a Quarantine Act, specifically to be compliant with the 1969 IHR.
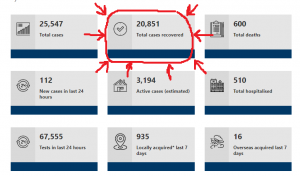
8. Australia Also Complies With IHR

Australia’s International Health Obligations
The International Health Regulations (2005) (IHR) are designed to prevent the international spread of infectious diseases while avoiding interference with international traffic and trade. As a Member State of the World Health Organization (WHO), Australia is obliged to comply with the IHR.
What are the International Health Regulations (2005)?
The IHR are an international legal instrument that is binding on 196 countries across the globe, including all Member States of the WHO. Their aim is to help the international community prevent and respond to acute public health risks that have the potential to cross borders and threaten people worldwide.
The IHR, which entered into force on 15 June 2007, require countries to report certain disease outbreaks and public health events to the WHO. Building on the unique experience of the WHO in global disease surveillance, alert and response, the IHR define the rights and obligations of countries to report public health events, and establish a number of procedures that the WHO must follow in its work to uphold global public health security.
Australia also must comply with the International Health Regulations of 2005. Of course, we must ask WHY these politicians are willingly handing over national sovereignty.
9. World Health Assembly (1995)
There were some changes in the 1995 version. However, I haven’t been able to find a version of it online. In any event, since the 2005 version is in effect, that matters more.
10. Foreword Of 2005 IHR Guide

FOREWORD
A central and historic responsibility for the World Health Organization (WHO) has been the management of the global regime for the control of the international spread of disease. Under Articles 21(a) and 22, the Constitution of WHO confers upon the World Health Assembly the authority to adopt regulations “designed to prevent the international spread of disease” which, after adoption by the Health Assembly, enter into force for all WHO Member States that do not affirmatively opt out of them within a specified time period.
A quote from the foreword of the 2005 edition of the International Health Regulations. No comment needed here.
There are 3 versions of the IHR: (a) 1969; (b) 1995; and (c) 2005. It’s predecessor was the International Sanitation Regulations, created in 1951.
The 2005 document still appears to be in place.
11. Int’l Health Regulations Legally Binding
What are the International Health Regulations?
.
The International Health Regulations (2005), or IHR (2005), represents a binding international legal agreement involving 196 countries across the globe, including all the Member States of WHO. Their aim is to help the international community prevent and respond to acute public health risks that have the potential to cross borders and threaten people worldwide. The purpose and scope of the IHR (2005) is to prevent, protect against, control and provide a public health response to the international spread of disease in ways that are commensurate with and restricted to public health risks, and which avoid unnecessary interference with international traffic and trade.
In case this wasn’t clear from the last several sections, the international health regulations ARE in fact, legally binding on all member states.
12. Canada A Party To 2005 IHR

APPENDIX 1
STATES PARTIES TO THE INTERNATIONAL HEALTH
REGULATIONS (2005) 1
Except as otherwise indicated, the International Health Regulations (2005) entered into force on
15 June 2007 for the following States:
Afghanistan, Albania, Algeria, Andorra, Angola, Antigua and Barbuda, Argentina, Armenia, Australia, Austria, Azerbaijan, Bahamas, Bahrain, Bangladesh, Barbados, Belarus, Belgium, Belize, Benin, Bhutan, Bolivia (Plurinational State of), Bosnia and Herzegovina, Botswana, Brazil, Brunei Darussalam, Bulgaria, Burkina Faso, Burundi, Cabo Verde, Cambodia, Cameroon, Canada, Central African Republic, Chad, Chile, China, Colombia, Comoros, Congo, Cook Islands….
Appendix I, on page 59, lists all of the parties to the International Health Regulations.
13. Constitution Of World Health Org.

Article 21
The Health Assembly shall have authority to adopt regulations concerning:
(a) sanitary and quarantine requirements and other procedures designed to prevent the international spread of disease;
(b) nomenclatures with respect to diseases, causes of death and public health practices;
(c) standards with respect to diagnostic procedures for international use;
(d) standards with respect to the safety, purity and potency of biological, pharmaceutical and similar products moving in international commerce;
(e) advertising and labelling of biological, pharmaceutical and similar products moving in international commerce.
Article 22
Regulations adopted pursuant to Article 21 shall come into force for all Members after due notice has been given of their adoption by the Health Assembly except for such Members as may notify the Director-General of rejection or reservations within the period stated in the notice.
Article 23
The Health Assembly shall have authority to make recommendations to Members with respect to any matter within the competence of the Organization.
Article 33
The Director-General or his representative may establish a procedure by agreement with Members, permitting him, for the purpose of discharging his duties, to have direct access to their various departments, especially to their health administrations and to national health organizations, governmental or non-governmental. He may also establish direct relations with international organizations whose activities come within the competence of the Organization. He shall keep regional offices informed on all matters involving their respective areas.
CHAPTER XV – LEGAL CAPACITY, PRIVILEGES AND IMMUNITIES
Article 66
The Organization shall enjoy in the territory of each Member such legal capacity as may be necessary for the fulfilment of its objective and for the exercise of its functions.
Article 67
(a) The Organization shall enjoy in the territory of each Member such privileges and immunities as may be necessary for the fulfilment of its objective and for the exercise of its functions.
(b) Representatives of Members, persons designated to serve on the Board and technical and administrative personnel of the Organization shall similarly enjoy such privileges and immunities as are necessary for the independent exercise of their functions in connexion with the Organization.
Article 68
Such legal capacity, privileges and immunities shall be defined in a separate agreement to be prepared by the Organization in consultation with the Secretary-General of the United Nations and concluded between the Members.
CHAPTER XVI – RELATIONS WITH OTHER ORGANIZATIONS
Article 69
The Organization shall be brought into relation with the United Nations as one of the specialized agencies referred to in Article 57 of the Charter of the United Nations. The agreement or agreements bringing the Organization into relation with the United Nations shall be subject to approval by a two thirds vote of the Health Assembly.
https://apps.who.int/gb/bd/pdf_files/BD_49th-en.pdf#page=7
The Constitution of the World Health Organization is listed in this book of basic documents. To sum up some of the main points:
(a) WHO has the authority to set regulation on quarantine matters
(b) WHO has authority over pharmaceutical matters
(c) WHO and its staff have legal indemnification
(d) WHO and its staff have access to national health data.
14. Quarantine Act, Ottawa Adopting IHR (2005)
The Paul Martin Liberals introduced Bill C-12, commonly known as the “Quarantine Act”. It passed 249-54, with only the Bloc Quebecois voting against it. It’s not a stretch to see what this was: the Federal Government domestically implementing regulations required by a supra-national body.
https://www.ourcommons.ca/DocumentViewer/en/38-1/HESA/report-2/
https://www.ourcommons.ca/DocumentViewer/en/38-1/HESA/meeting-4/notice
quarantine.act.dec.8.2004.hearings
Must be quite the coincidence that the Federal Government was conducting hearings into passing a Quarantine Act, around the same time the World Health Organization was updating its International Health Regulations. It’s almost like they coordinated on it.
Of course, there have been some modifications to the Quarantine Act over the years, but same principles remain intact.
15. Covid World Health Assembly (2020)

At the historic 73rd World Health Assembly in May, Member States adopted a landmark resolution that called on WHO to initiate an independent and comprehensive evaluation of the lessons learned from the international health response to COVID-19.
Noting resolution EB146.R10 (2020) on strengthening preparedness for health emergencies: implementation of the International Health Regulations (2005), and reiterating the obligation for all States parties to fully implement and comply with the International Health Regulations (2005);
That’s right, the May 2020 Convention called for all nations to comply with their MANDATORY obligations under the IHR. “Obligation” means that it isn’t optional.
1. CALLS FOR, in the spirit of unity and solidarity, the intensification of cooperation and collaboration at all levels in order to contain and control the COVID-19 pandemic and mitigate its impact;
2. ACKNOWLEDGES the key leadership role of WHO and the fundamental role of the United Nations system in catalysing and coordinating the comprehensive global response to the COVID-19 pandemic, and the central efforts of Member States therein;
3. EXPRESSES its highest appreciation of, and support for, the dedication, efforts and sacrifices, above and beyond the call of duty of health professionals, health workers and other relevant frontline workers, as well as the WHO Secretariat, in responding to the COVID-19 pandemic;
4. CALLS FOR the universal, timely and equitable access to, and fair distribution of, all quality, safe, efficacious and affordable essential health technologies and products, including their components and precursors, that are required in the response to the COVID-19 pandemic as a global priority, and the urgent removal of unjustified obstacles thereto, consistent with the provisions of relevant international treaties, including the provisions of the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement) and the flexibilities within the Doha Declaration on the TRIPS Agreement and Public Health;
9. REQUESTS the Director-General:
(4) to provide support to countries upon their request, in accordance with their national context, in support of the continued safe functioning of the health system in all relevant aspects necessary for an effective public health response to the COVID-19 pandemic and other ongoing epidemics, and the uninterrupted and safe provision of population- and individual-level services, for, among other matters: communicable diseases, including through undisrupted vaccination programmes, and for neglected tropical diseases, noncommunicable diseases, mental health, mother and child health and sexual and reproductive health; and to promote improved nutrition for women and children;
Yes, they absolutely had to throw in a pledge to keep abortion accessible to all. If this “virus” is so deadly, why exactly are we pushing to kill more kids, and at a faster rate?
9. REQUESTS the Director-General:
(5) to support countries, upon request, in developing, implementing and adapting relevant national response plans to COVID-19, by developing, disseminating and updating normative products and technical guidance, learning tools, data and scientific evidence for COVID-19 responses, including to counter misinformation and disinformation, as well as malicious cyber activities, and to continue to work against substandard and falsified medicines and medical products;
Countering “misinformation and disinformation”? One can’t help but be reminded of Objective 17(c) of the UN Global Migration Compact, which called for defunding, and ultimately silencing critics of the population replacement agenda. Presumably this time those people are the ones questioning the official narrative.
https://www.who.int/about/governance/world-health-assembly/seventy-third-world-health-assembly
ihr.may.2020.who.convention.free.speech
Aside from the self-congratulatory nature of the resolution, it is actually quite alarming, some of the contents within it.
16. All An Excuse To Implement Changes
To repeat a point made earlier, the International Health Regulations that the WHO puts out are MANDATORY. They are binding on all member states, which Canada is one.
The Quarantine Act brought in by the Martin Liberals seems like a way to domestically implement what the WHO was doing globally. The timing is too coincidental, and they all speak the same. The Quarantine Act also specifies that it is binding both on Ottawa, and the Provinces.
Given the lies and contradictions coming from our officials, nothing they say can be trusted. All of this comes across as a means to implement a larger social agenda.
It’s not limited to Canada either. Two of the examples posted are Australia and New Zealand, nations similar in many ways to us.