
1. Other Articles On CV “Planned-emic”
CLICK HERE, for #0: Theresa Tam; archives; articles; lobbying.
CLICK HERE, for #1: piece on Bill Gates, Pirbright, depopulation.
CLICK HERE, for #2: Coronavirus research at U of Saskatchewan.
CLICK HERE, for #3: Gates; WHO, ID2020; GAVI; Vaccines.
CLICK HERE, for #4: Gates using proxies to push vaxx agenda.
CLICK HERE, for #5: Crestview Strategy, GAVI’s lobbying firm.
CLICK HERE, for #6: people GAVI/Crestview lobbied follow Gates.
2. HESA Submissions, Evidence, Reports
Submissions Lodged
hesa.Structural.Genomics.Consortium.submission
hesa.Medicines.Patent.Pool.2018
hesa.Doctors.Without.Borders.2018
hesa.Canadian.Institutes.Of.Health.Research.2018
hesa.Fowke.Keith.University.Manitoba.2018
hesa.University.College.London.drug.prices.2018
hesa.Drugs.For.Neglected.Diseases.Initiative.2018
hesa.Moon.Suerie.2018
hesa.Yusuf.Salim.mcmaster
hesa.FIND.tb.alliance.gates.gavi.unitaid
hesa.Vlassoff.Carol.2018
hesa.Universities.Allied.For.Essential.Medecines.2018
hesa.Bruyere.Research.Institute.2018
hesa.Molyneux.David.2018
LINK To Parliamentary Study Main Page
3. Federally Funded Health Research: M-132
For a speech on passing M-132.
The text is below
Motion Text
That the Standing Committee on Health be instructed to undertake a study on ways of increasing benefits to the public resulting from federally funded health research, with the goals of lowering drugs costs and increasing access to medicines, both in Canada and globally; and that the Committee report its findings and recommendations to the House no later than one year from the time this motion is adopted.
4. Parliamentary Committee Meetings
Dates Of Meetings
Thursday, September 27, 2018
Hesa.2018.September.27.evidence.transcript
Tuesday, October 2, 2018
Hesa.2018.October.2.evidence.transcript
Thursday, October 4, 2018
Hesa.2018.October.4.evidence.transcript
Tuesday, October 16, 2018
Hesa.2018.October.16.evidence.transcript
Thursday, October 18, 2018
Hesa.2018.October.18th.evidence.transcript
Tuesday, October 23, 2018
Hesa.2018.October.23.evidence.transcript
Thursday, October 25, 2018
Hesa.2018.October.25.evidence.transcript
5. Reports Released To The Commons

In Canada and around the world, there is rising concern that innovative drugs produced by pharmaceutical companies are no longer affordable and are placing increasing strain on health care budgets. Policy makers have begun to examine ways that public funding for pharmaceutical research and development could address this issue. On 8 November 2017, the House of Commons adopted Private Members’ Business M-132, which requested that the House of Commons Standing Committee on Health (the Committee) “undertake a study on ways of increasing benefits to the public resulting from federally funded research, with the goals of lowering drug costs and increasing access to medicines, both in Canada and globally.”
On 16 and 18 October 2018, the Committee held two meetings as part of this study and heard from a range of witnesses including health researchers, health research funding organizations, patient groups and civil society organizations. Drawing on witness testimony and written submissions, this report examines the role the federal government can play in fostering pharmaceutical research and development both in Canada and globally to ensure that pharmaceutical drugs are accessible and affordable.
Note: Recommendations can be found starting at page 20 in the 2018 report released to the House of Commons.
HOUSE OF COMMONS STANDING COMMITTEE ON HEALTH CALLS ON THE GOVERNMENT OF CANADA TO FOSTER PHARMACEUTICAL RESEARCH AND DEVELOPMENT BOTH IN CANADA AND GLOBALLY THROUGH OPEN SCIENCE
Ottawa, November 26, 2018 –Bill Casey, Chair of the House of Commons Standing Committee on Health, presented the Committee’s twentieth report today entitled, Towards Open Science: Promoting Innovation in Pharmaceutical Research and Development and Access to Affordable Medications both in Canada and Abroad.
The Committee’s study is in response to Member of Parliament Raj Saini’s Private Members’ Motion M-132, which requested that the Committee, “undertake a study on ways of increasing benefits to the public resulting from federally funded research, with the goals of lowering drug costs and increasing access to medicines, both in Canada and globally.”
In presenting the report to the House, Chair Bill Casey highlighted that “in our testimony, we heard loud and clear that more needs to be done to strengthen research and innovation in Canada. I thank Mr. Saini for bringing forth M-132, and for his efforts in ensuring that the Health Committee can hear why Canada must continue to be a leader in this field.”
Drawing on witness testimony heard over the course of two meetings held on 16 and 18 October 2018 and on 23 written submissions, the Committee’s report examines how increased federal investment in health research, across the continuum from fundamental to clinical research, would support the development of new medicines. However, witnesses also emphasized the importance of ensuring that federal funding in pharmaceutical research and development must also result in the creation of drugs that are affordable in Canada and abroad. Witnesses suggested that this could be achieved by fostering the creation of innovative models of pharmaceutical research that prioritize open science in both the development of new drugs and the repurposing of existing drugs. Witnesses explained that the Government of Canada could lead the way by developing a framework that sets priorities for pharmaceutical research and development and promotes open science through collaboration and leveraging of funding across governments, universities, health charities and private industry.
The Committee agrees with these findings and has included in its report nine recommendations that it believes will support the transformation of pharmaceutical research and development in Canada.
The announcement of the press release is here
Recommendation 1
That the Government of Canada create a specific funding mechanism for the development of clinical trial research and infrastructure in Canada through the Canadian Institutes of Health Research.
.
Recommendation 2
That the Government of Canada increase its funding for clinical trial research and infrastructure in Canada to 10% of the Canadian Institutes of Health Research’s budget to be on par with jurisdictions leading in this area, such as the United Kingdom and the United States.
.
Recommendation 3
That the Government of Canada explore ways to incentivize clinical trial research in Canada for pharmaceutical drugs and incentivize and support the production of those drugs in Canada at an advantaged price for Canada and provide venture capital for the proponent.
.
Recommendation 4
That the Canadian Institutes of Health Research attach a Global Access Licensing requirement to recipients of its research funding that wish to commercialize their research findings.
.
Recommendation 5
That the Canadian Institutes of Health Research include in its existing research and development programs support for the development of open science models of drug discovery.
.
Recommendation 6
That the Canadian Institutes of Health Research develop a framework for open science that supports collaboration and the leveraging of research funding among different partners in pharmaceutical research and development, including health charities, universities, governments, and private industry.
.
Recommendation 7
That Health Canada develop regulatory incentives for pharmaceutical companies that commit to open access to their research data and affordable prices for their products.
.
Recommendation 8
That the Government of Canada undertake a strategic review of its health-related research funding priorities across departments and agencies to enhance coordination, including Health Canada, Public Health Agency of Canada, Canadian Institutes of Health Research, Global Affairs Canada, and Innovation, Science and Economic Development Canada.
.
Recommendation 9
That the Government of Canada explore the feasibility of the public manufacturing of generic medicines.
In the follow-up report, the recommendations were formally adopted.
REPORTS TO PARLIAMENT
hesa.november.2018.report.to.parliament
hesa.government.response.march.2019
6. Committee Members
As provided by the report, these are the names and ranks of the Committee.
STANDING COMMITTEE ON HEALTH
CHAIR
- Bill Casey
VICE-CHAIRS
MEMBERS
- Ramez Ayoub
- Doug Eyolfson
- Raj Grewal
- Ben Lobb
- Ron McKinnon
- John Oliver (Parliamentary Secretary — Non-Voting Member)
- Sonia Sidhu
- Len Webber
OTHER MEMBERS OF PARLIAMENT WHO PARTICIPATED
- Randy Boissonnault
- Terry Duguid
- Randy Hoback
- Tom Kmiec
- Christine Moore
- Raj Saini (lobbied by GAVI)
- Dave Van Kesteren
CLERK OF THE COMMITTEE
- Marie-Hélène Sauvé
Why is the list of the Committee Members here? Well, once you see who some of the connections are, it will likely make the report findings a lot more suspicious.
7. Committee Members & Pharma Lobbying





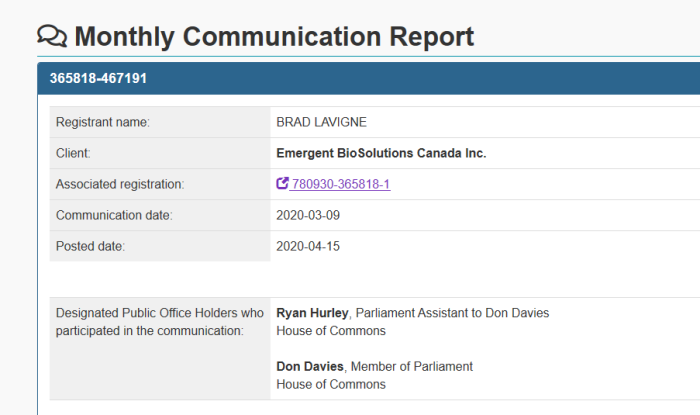


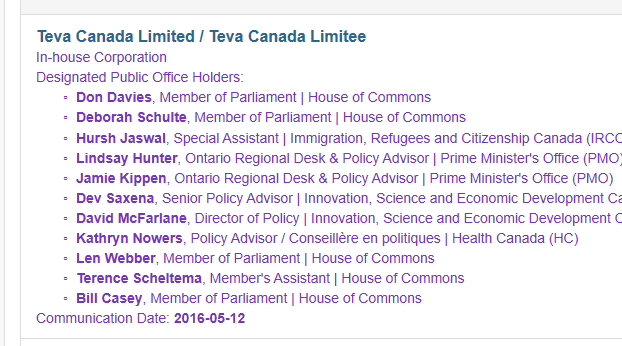




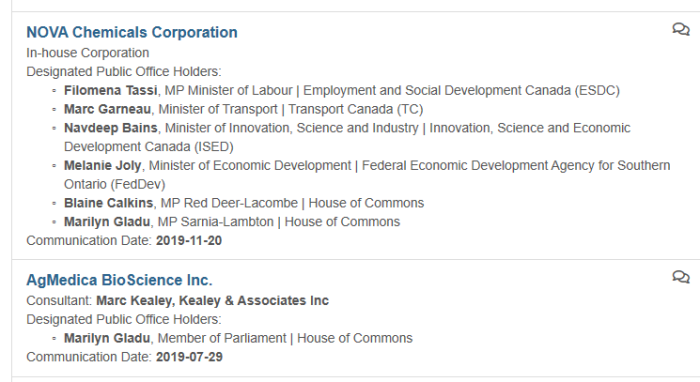

The above screenshots came from information provided in the Office of the Lobbying Commissioner of Canada. These are far from exhaustive, but show a snapshot at the lobbying that is going on in Canada. Members of this Parliamentary Committee are being lobbied by various drug companies. It’s not difficult to see that this is done in order to influence them.
8. Conflict Of Interest Here
The same committee members who are recommending that Canada undertake more research for pharmaceuticals are the same ones who are being lobbied by pharmaceutical companies. It’s not difficult to piece it together.
